Unit 1 Notes
Topic 1: Biology Review and an Introduction to Statistics
Science
- at the heart of science is inquiry
- inquiry - search for information and explanations
two main steps:
- making observations
- forming a hypothesis
Making Observations
- describes natural structures and processes through observations and analysis of data
- data - recorded observations
- qualitative - observations with senses
- quantitative - measured using instruments
- ==inductive reasoning== - derive generalizations based on a large number of specific observations
- ==deductive reasoning== - specific results that are derived from general premises
Forming Hypotheses
==hypothesis== - a testable prediction based on observations
- results can either support or refute the hypothesis
- NEVER say the hypothesis is correct or true
Null and Alternative Hypotheses
always start with a null hypothesis (H0)
- ==null hypothesis== - a hypothesis which the researcher tries to disprove, reject, or nullify
- i.e. there will be no difference in headache relief between individuals who take Tylenol and those who don’t
after the null, list all the alternative hypotheses (H1, H2, H3…)
- ==alternative hypothesis== - a hypothesis that may be supported by the data
Scientific Method
- most scientific inquiries do not follow a perfectly structured form
scientists can be working with the wrong hypothesis and have to redirect research
Hypothesis vs Theory vs Law
| ==Hypothesis== - a testable prediction | ==Theory== - summarizes a group of hypotheses | ==Law== - statement of fact (mathematical formula) |
|---|---|---|
| - tested by experiment or continued observation | - broader in scope | - describes an observation, not “how” or “why” |
| - can be disproven, but cannot be proved true | - a new hypothesis can be generated from it | - generally accepted to be true and universal |
| - supported by LOTS of evidence | ||
| - NEVER becomes a law |
Experiments
start with an observation and a hypothesis
use control groups and experimental groups
- well designed experiments should include:
- independent variable
- dependent variable
- control group (+ or -)
- constants
- multiple trials (in the scientific community 3 is the accepted minimum number of trials)
Variables vs Constants
- A ==variable== is something that is changed in the experiment
- A ==constant== is something that does not change throughout the experiment
- ==independent variable== - the one factor that is changed by the person running the experiment
- represents a quantity that is being manipulated during the experiment
- ==dependent variable== - the factor which is measured in the experiment
- represents the quantity whose value depends on how the independent variable is manipulated
Experimental Controls
- Control group - expected results
- Experimental results - experimental results
- COMPARE the two
Control Groups
- controls are essential elements of an experiment:
- they help eliminate experimental errors and biases of researchers
- results of the control experiments validate statistical analysis of the experiment
- reliability of the experiment increases
NOTE: controls are NOT constants
Types of Control Groups
- There are two types of control groups:
| ==Positive Control Group== | ==Negative Control Group== |
|---|---|
| - group is not exposed to the experimental treatment or independent variable, but it IS exposed to a treatment known to produce effect | - group is not exposed to any treatment or exposed to a treatment that is known to have NO effect |
| - ensures there is an effect when there should be an effect | - ensures that there is no effect when there should be no effect; nothing is expected to happen |
| - if the positive control group does not produce the expected result, there may be something wrong with the experimental procedure | - a negative control can be a different way of establishing a baseline |
| - scientists use positive controls when they are trying to induce a positive result | - used to ensure that no confounding/outside variable has affected the results, or to factor in any likely sources of bias |
Statistics
- scientists typically collect data on a sample of a population
- the first step in analysis is to graph the data and examine the distribution
- typical data will show normal distribution
- bell shaped curve
Measures of Central Tendencies
- ==central tendencies== - the center of the distribution can be described by the mean, median, and mode
- ==mean== - the average of the data set
- ==median== - the middle number/value of the data set
- ==mode== - the value that occurs most often in a data set
- not usually used to measure central tendency
Measures of Variability
==variability== - the measure of how far a data set diverges from the central tendency
- measured by range and standard deviation
==range== - the difference between the largest and smallest value of a data set
- large range = greater variability
- smaller range = smaller variability
- often used in conjunction with standard deviation
==standard deviation== - a measure of how spread out the data is from the mean
- low standard deviation - the data is closer to the mean
- the independent variable is likely causing changes
- high standard deviation = the data is farther from the mean
- factors other than the independent variable are likely causing changes
- 1 standard deviation from the mean in either direction on the horizontal axis represents 68% of the data
- 2 standard deviation from the mean in either direction on the horizontal axis represents 95% of the data
- 3 standard deviation from the mean in either direction on the horizontal axis represents 99% of the data
- formula:
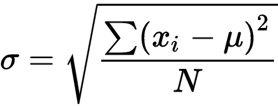
there are four steps to solve for standard deviation
- find the mean
- determine the deviation from the mean for each data point and square it
- calculate degrees of freedom (n-1), n is the sample size
- put it all together and calculate s
Standard Error of the Mean
used to determine the precision of and confidence in the mean value
based on:
- standard deviation
- the number of data points
low standard error = increase in confidence
- commonly given as +/- 2 SEM (95% confidence)
formula:
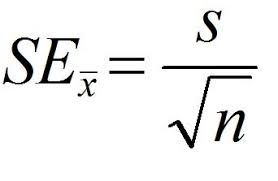
Error Bars
- standard error bars are often added to graphs
- if error bars overlap, the difference is not significant
- if error bars do not overlap, the difference may be significant
Topic 1: Structure of Water and Hydrogen Bonding
Chemistry Review
| ==Matter== | ==Element== | ==Compound== |
|---|---|---|
| - anything that takes up space and has mass | - a substance that can’t be broken down into other substances in a chemical reaction | - a substance consisting of tow or more elements combined in a fixed ratio |
| - i.e. rocks, metals, oils, organisms, etc | - 92 elements occur in nature | - i.e. water |
==essential element==s - of the 92 naturally occurring elements, 20-25% are essential to survive and reproduce
- CHOPN make up 96% of living matter
t==race elements== - of the 92 naturally occurring elements, these are required by an organism in very small quantities
==atomic mass== - number of protons plus number of neutrons averaged out over all naturally occurring isotopes of an element
Periodic Table
- elements in the same period have the same total number of electron shells
- elements in the same group have the same number of valence electrons
Types of Bonds
elements want to be stable
achieve this by forming chemical bonds with other elements
- ==octet rule== - elements will gain, lose, or share electrons to complete their valence shell and become stable
==chemical bonds== - a attraction between two atoms, resulting from the sharing or transferring of valence electrons
==electronegativity== - the measure of an atom’s ability to attract electrons to itself
==covalent bonds== - when two or more atoms share electrons (usually between nonmetals)
forms molecules and compounds
- single bond - 1 pair of shared electrons
- double bond - 2 pairs of shared electrons
- triple bond - 3 pairs of shared electrons
there are two types of covalent bonds: non-polar covalent bonds and polar covalent bonds
- ==non-polar covalent== - electrons are shared equally between two atoms
- ==polar covalent== - electrons are not shared equally between two atoms
- unequal sharing of electrons results in partial charges of the O and Hs of a water molecule
==ionic bonds== - the attraction between oppositely charged atoms (ions)
usually between a metal and a nonmetal
forms ionic compounds and salts
occurs when there is a transfer of electrons
==hydrogen bonds== - the partially positive hydrogen atom in one polar covalent molecule will be attracted to an electronegative atom in another polar covalent molecule
intermolecular bond - bond that forms between molecules
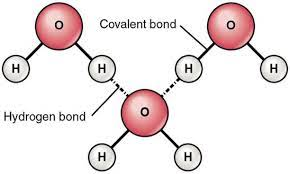
Properties of Water
- ==polarity==
- unequal sharing of electrons makes water a polar molecule
- ==cohesion==
- attraction of molecules to other molecules of the same kind
- hydrogen bonds between H2O molecules hold them together and increase cohesive forces
- allows for the transport of water and nutrients against gravity in plants
- responsible for surface tension = allow liquids to resist external force
- ==adhesion==
- the clinging of one molecule to a different molecule
- due to polarity of H2O
- in plants, this allows for water to stick to the cell walls to resist the downward pull of gravity
- the clinging of one molecule to a different molecule
- ==capillary action==
- the upward movement of water due to the forces of cohesion, adhesion, and surface tension
- occurs when adhesion is greater than cohesion
- important for transport of water and nutrients in plants
- temperature control
- ==high specific heat== - water resists changes in temperature
- how? hydrogen bonds!
- heat must be absorbed to break H bonds, but heat is released when H bonds form
- importance of high specific heat:
- moderates air temperature
- large bodies of water can absorb heat in the daytime and release heat at night
- stabilizes ocean temperature
- benefits marine life
- organisms can resist changes in their own internal temperature
- ==evaporative cooling== - water has a high heat of vaporization
- the molecules with the highest kinetic energy leave as gas
- importance of evaporative cooling
- moderates earth’s climate
- stabilizes temperatures in lakes and ponds
- prevents terrestrial organisms from overheating (think sweating in humans)
- density
- as water solidifies it expands and becomes less dense
- due to hydrogen bonds
- when cooled, water molecules move too slowly to break the bonds
- allows marine life to survive under floating ice sheets
- as water solidifies it expands and becomes less dense
- solvent
- dissolving agent in a solution
- water is a versatile solvent
- its polar molecules are attracted to ions and other polar molecules it can form hydrogen bonds with
- ==solution== - homogenous mix of two or more substances
- ==solvent== - dissolving agent in a solution
- ==solute== - substance that is dissolved
- “like dissolves like”
- water can interact with sugars or proteins containing many oxygen and hydrogen atoms
- water will form hydrogen bonds with the sugar or protein to dissolve it
- ionic compounds
- the partially negative oxygen in water will interact with a positive atom
- the partially positive hydrogen in water will interact with a negative atom
Topic 2: Elements of Life
Carbon
- ==organic chemistry== - the study of compounds with covalently bonded carbon
- ==organic compounds== - compounds that contain carbon and hydrogen
- carbon has 4 valence electrons
- carbon can form single, double, or triple covalent bonds
- a single carbon can form up to four covalent bonds
- can form long chains
- most commonly formed with hydrogen, oxygen, and nitrogen
- the type and number of covalent bonds carbon forms with other atoms affects the length of the carbon chain and shape of the molecule
Carbon Chains
- carbon can use its valence electrons to form covalent bonds to other carbons
- this links carbons into a chain
- ==hydrocarbons== - organic molecules consisting only of carbon and hydrogen
- a simple framework for more complex molecules
- carbon chains form the skeletons of most organic molecules
- skeletons can vary in:
- length
- branching
- double bond position
- presence of rings
- many regions of a cells organic molecules contain hydrocarbons
- ==functional groups==
- chemical groups attached to the carbon skeleton that participate in chemical reactions
- MEMORIZE
- hydroxyl group
- carbonyl group
- carboxyl group
- amino group
- sulfhydryl group
- methyl group
- phosphate group
Topic 3: Introduction to Biological Macromolecules
Molecular Diversity due to Carbon
- variations in carbon skeletons allows for molecular diversity
- carbon can form large chains known as macromolecules
- four classes of macromolecules (molecules made of smaller subunits):
- polymers
- carbohydrates
- proteins
- nucleic acids
- lipids → does not include true polymers and are hydrophobic molecules
- nitrogen is important in building proteins
Formation and Breakdown of Macromolecules
- ==polymers== - chain-like macromolecules of similar or identical repeating units that are covalently bonded together
- ==monomers== - the repeating units that make up polymers
- ==dehydration reaction== - bonds two monomers form with the loss of water
- the -OH of one monomer bonds to the -H of another monomer forming water which is then released
- A + B → AB + H2O
- ==hydrolysis reaction== - breaks the bonds in a polymer by adding water
- one -H of the water bonds to one monomer and the remaining -OH of the water attaches to the other monomer
- AB + H2O → A + B