Reactions of Organic Compounds
Alkane reactions - alkanes are not very reactive due to being non-polar and having a low electron availability due to only having
- Takes something very reactive to react with alkanes.
Halogenation of an alkane
- Process of substituting a hydrogen
- C-H + 2X → C-X + HX
- Halogenation has to be catalyzed by light or heat
- What happens to the Halogen
- With light,
- Free radical
- Particle in which there is an unpaired electron
- VERY UNSTABLE
- Homolytic cleavage
- Breaking of a bond where each atom gets one of the electrons
- Free Radical Mechanism for Halogenation of Alkanes

- Initiation starts with molecules and ends with Free Radicles
- Propagation starts with a Free Radical and a Molecule ends with a different Free Radical and a Molecule
- Termination
- If the concentration of the molecule is much greater than the concentration of the halogen, mainly monohalogenated alkenes.
- If the concentration of the molecules is much less than the concentration of the halogen, mainly completely halogenated alkanes
- In both cases, di and trihalogenations occur as well
Halogenoalkane reactions
- Nucleophilic substitution
- Positive loving → electron-dense reactant
Oxidation of alcohols
- Primary alcohol
- Catalysts
- Dichromate / acidic environment
- heat and distill
- get an aldehyde
- or
- dichromate / acidic environment
- heat and reflux
- carboxylic acid
- Secondary
- dichromate / acidic environment
- heat and reflux
- ketone
- Tertiary
- dichromate / acidic environment
- heat and reflux
- no reaction
Esterification
- The condensation reaction between a carboxylic acid and an alcohol
- Water is a product
- Carboxylic acid + alcohol → HOH + Ester
- The hydroxyl group from the carboxylic acid and the hydrogen from alcohol come together.
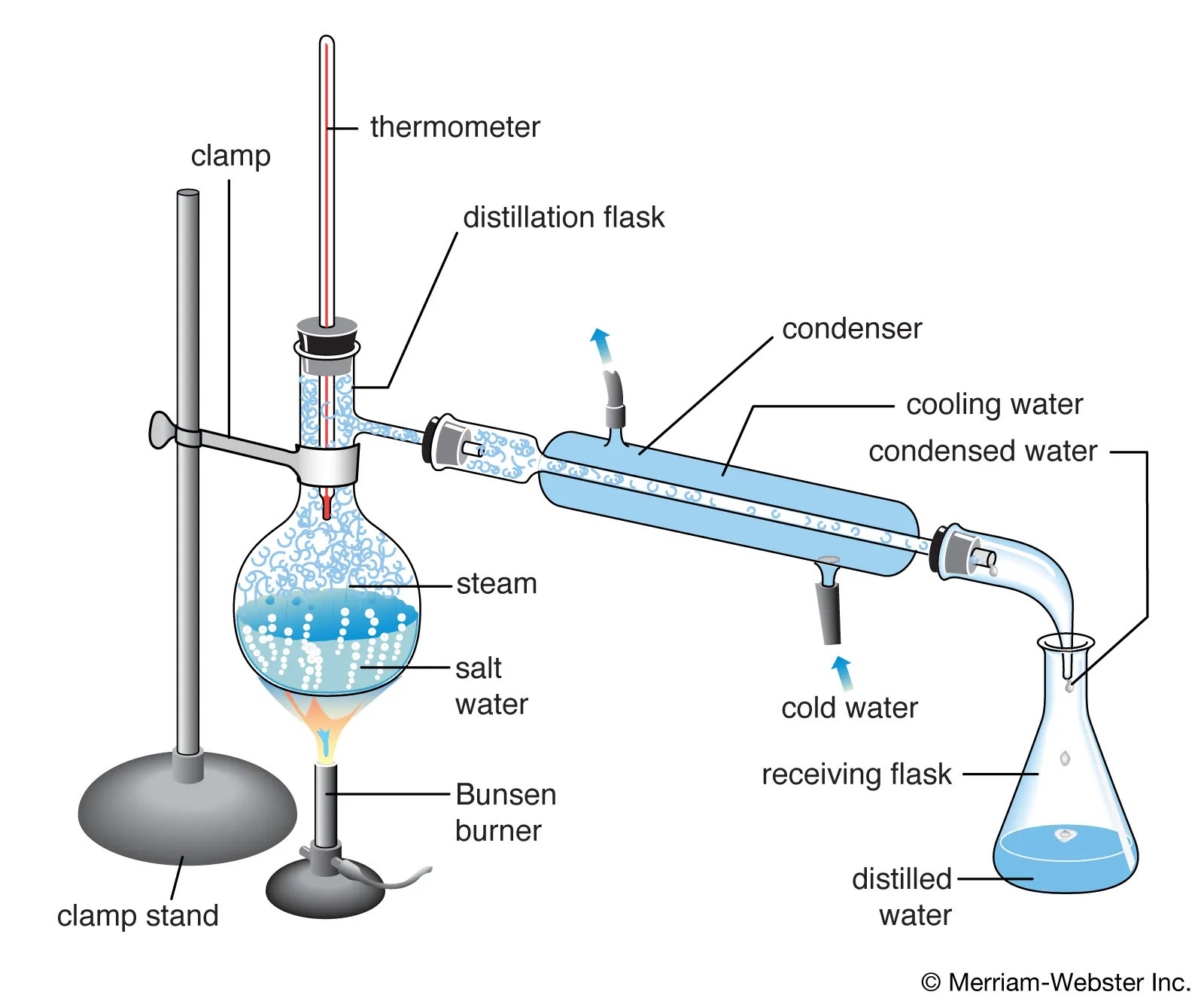
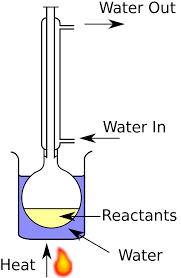
Distillation
Reflux
Practice
Determine the product(s) (Draw, name, and state conditions) for the oxidation of
3 - methyl pentane-1-ol
- C6H14O
- 3-methylpentanal
- Heat and distillation with hydrolyzed dichromate
- or
- Heat and reflux with hydrolyzed dichromate
- 3-methyl pentanoic acid
4- methyl heptane-4-ol
- No reaction it is a tertiary alcohol
hexane-3-ol
- C6H12O
- acidized dichromate and heat and reflux
- hexane-3-one
Complete Combustion
- Alkanes
- CnH2n+2 + O2 (g) → CO2(g) + H2O (g)
Incomplete Combustion (lack of oxygen)
- CnH2n+2 + O2 (g) →CO (g) + H2O (g)
Incomplete combustion (severe lack of oxygen)
- CnH2n+2 +O2 (g) → C (s) + H2O (g)
Alkenes are more reactive than alkanes because the electrons in the pi bond lie above and below the plane, making them more easily accessible than the electrons in the sigma bond.
Electrophilic addition:
- electron loving
- positive or partial positive substances
Hydrogenation
- metal catalyst
- \
- H2
Halogenation
- Capital X is used to represent a halogen
- two carbons with a sigma bond + X2 →
- →
Hydrohalogenation
- Hydrogenhaylide
- hydrogen on one and carbon on the other
Hydration
- adding water
Polymers
- means “many pieces”
- GIANT molecules with repeating units
- Hundreds of thousands of times
- Examples
- teflon, styrofoam, nylon, rubber, PVC,
Benzene
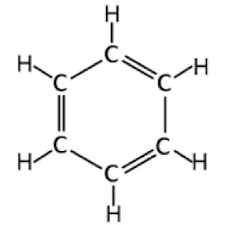
- Physical Properties of Benzenes
- In benzenes, all the bonds are the same length
- The shape of benzene is a regular hexagon
- The bond order of benzene is 1.5
- Chemical Properties of Benzene
- Benzens don’t undergo addition reactions like alkenes do
- The enthalpy of hydrogenation of benzene is lower than expected, which means that it is stable and has lower energy.
- Electrophilic (Electron loving) Substitution of Benzenes
- Electrophilic Substitution of Benzenes requires an acid catalyst
- One of the products of the Electrophilic substitution of benzene is water in the HOH form
- Halogenation of Benzenes
- The halogenation of benzenes needs an acid catalyst.
- Lewis Acid Definition of Acid and Bases
- Definition: A Lewis acid is a chemical species that can accept a pair of electrons to form a covalent bond.
- Examples of Lewis acids:
- Metal cations (e.g. Al3+, Fe2+, Zn2+)
- Boron trifluoride (BF3)
- Carbonyl compounds (e.g. ketones, aldehydes)
- Lewis acid-base adducts (e.g. H+ + NH3 → NH4+)
- \