2.3 Cell division and Differentiation
Cell Division (LO1)
The human body contains approximately 40 trillion cells.
Cells reproduce via division, which contributes to:
Tissue development/growth
Tissue renewal/replacement
Tissue regeneration/repair
Different tissues exhibit varying rates of cell division.
Normal cells vs Cancer cells:
Diseases such as neurodegeneration, autoimmune disorders, metabolic issues, cardiovascular diseases, and malignancies can affect normal cell function and division.
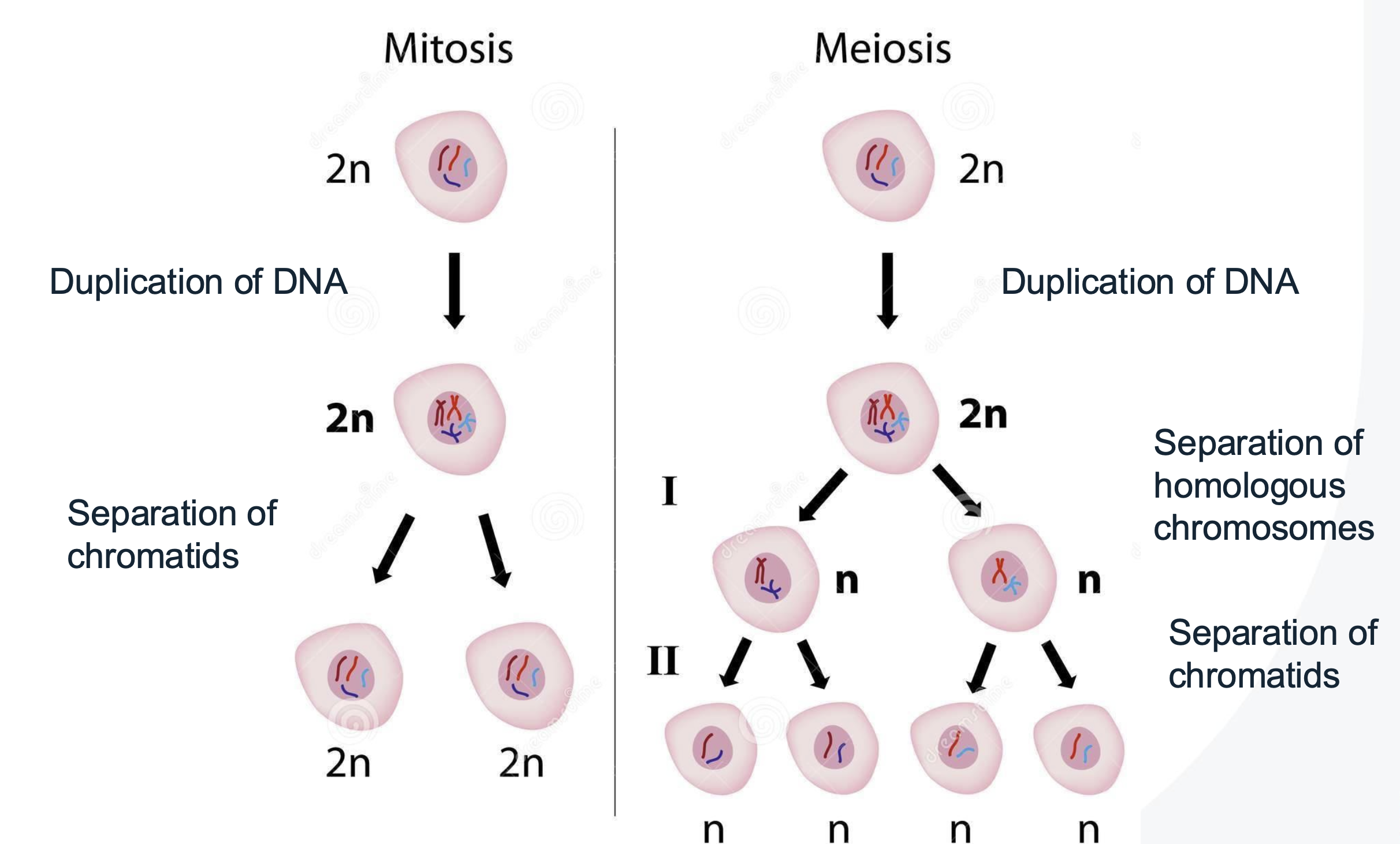
Mitosis
DNA content is duplicated
Cell divides into two identical diploid daughter cells

Diploid
paired chromosomes, one from each parents (2n)
Haploid
single set of chromosomes (n)
Cell Cycle
Interphase
Cells perfoming normal function
Not actively focused on division
can be indefinite
prepares for mitosis
Phases of Interphase
G1 phase:
Pre-mitosis preparation (8-12+ hours), normal cell function and generation of organelles.
S phase:
DNA replication and synthesis of histones (6-8 hours).
G2 phase:
Final preparations for mitosis (2-5 hours).
G0 phase:
Non-dividing state where cells function normally.
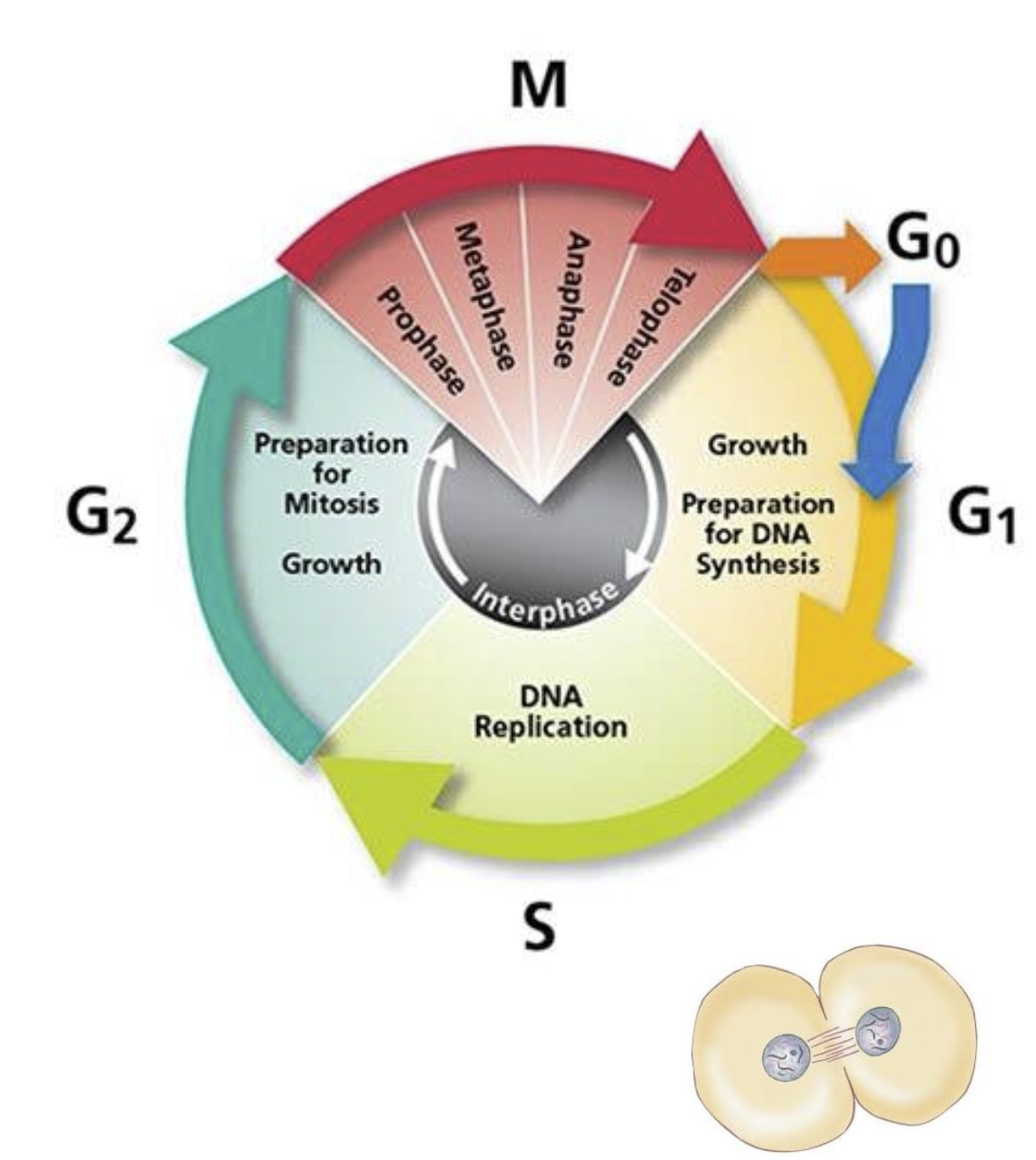
M Phase (Mitosis)
The M phase is the stage of the cell cycle where mitosis occurs, involving the division of the cell's nucleus and cytoplasm.
Results in two identical diploid daughter cells with replicated chromosomes.
Duration: Mitosis typically takes about 1-3 hours to complete.
Stages of Mitosis:
Prophase:
DNA coils and condenses; nuclear membrane disappears.
Centrosomes migrate to opposites sides of the cell.
Metaphase:
Chromosomes align in the center attached to spindle fibers.
Attached to microtubules held by centrosomes.
Anaphase:
Microtubules pull chromatids towards opposite poles.
Telophase:
Nuclear membranes reform; DNA uncoils.
Cytokinesis:
This is the physical separation of the cytoplasm into two daughter cells following mitosis.
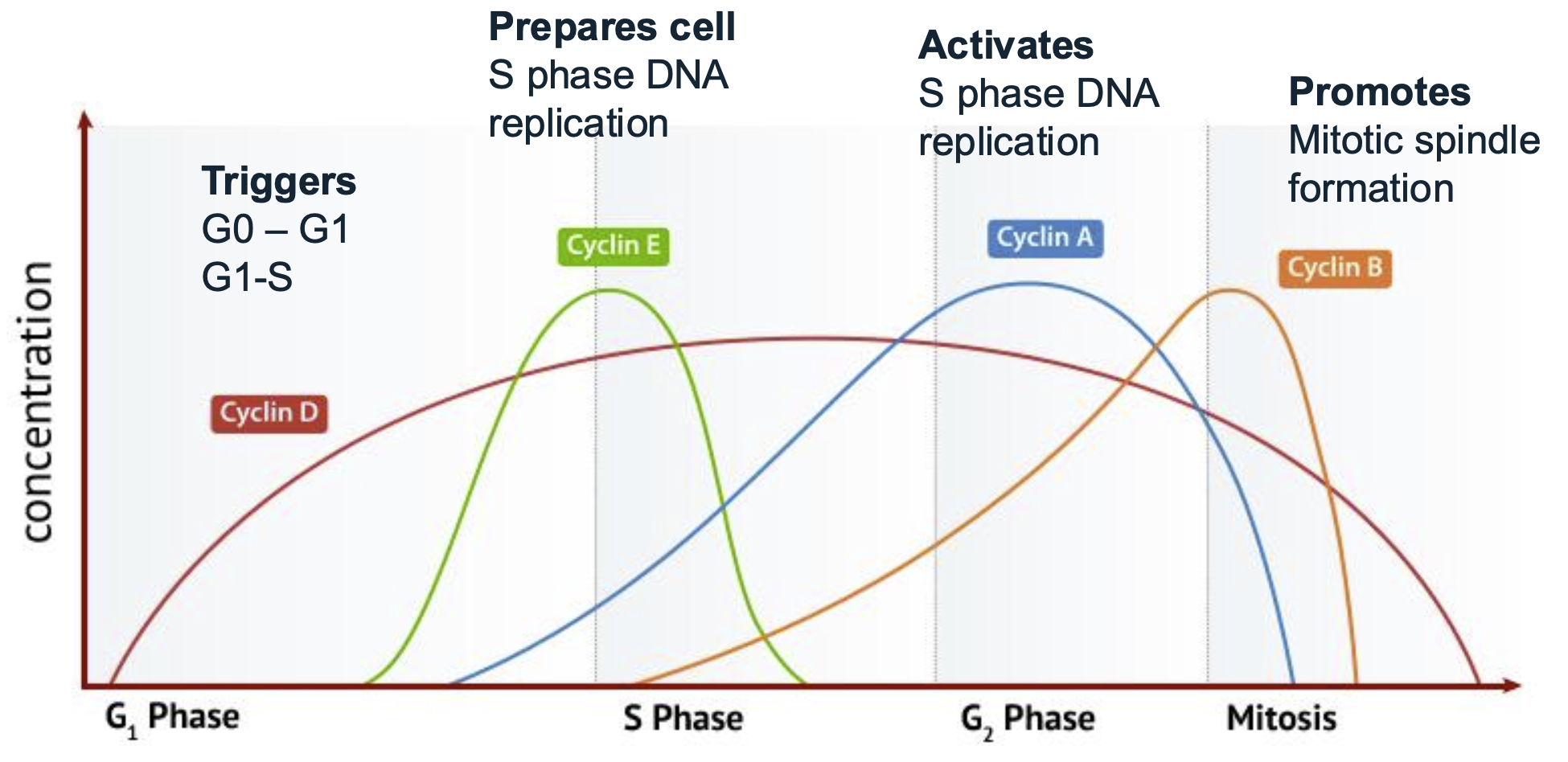
Cyclins
Cyclins are proteins that regulate the progression of the cell cycle by activating Cyclin-Dependent Kinases (CDKs).
Different types of cyclins correspond to specific phases of the cell cycle and trigger transitions between these phases.
CDKs are activated by cyclins to facilitate cellular functions necessary for cell cycle progression.
Cyclin D
Triggers G0-G1 and G1-S
Cyclin E
Prepares cells for S phase DNA relication
Cyclin A
Activates S phase during DNA replication
Cyclin B
Mitotic spindle formation
Cyclin Dependent kinases
Cyclin-Dependent Kinases (CDKs) are enzymes that play a crucial role in regulating the cell cycle. CDKs are activated by cyclins, and together they facilitate the progression of the cell cycle by phosphorylating target proteins necessary for various cellular functions. Different types of CDKs (e.g., CDK2, CDK4/6) are associated with specific phases of the cell cycle, and they work in tandem with corresponding cyclins to ensure proper timing and coordination of cell division.
Cyclin A requires CDK 2/1 for activation
Cyclin B requires CDK 1 for activation
Cyclin D requires CDK 2/4/6 for activation
Cyclin E requires CDK 2
Cyclin Dependent Kinase Inhibitors (CDKIs)
CDKIs are proteins that inhibit the activity of cyclin-dependent kinases (CDKs), thereby regulating the cell cycle progression.
They play a vital role in maintaining the balance of cell division and preventing uncontrolled cell growth.
Examples of CDKIs include:
p16: a tumor suppressor protein that inhibits CDK4 and CDK6, thereby preventing the transition from the G1 phase to the S phase of the cell cycle.
p14: p14 is another important CDKI that inhibits cyclin E-CDK2 activity, thereby regulating the progression from G1 to S phase and maintaining proper cell cycle control.
p15: p15: p15 is a cyclin-dependent kinase inhibitor (CDKI) that has been shown to regulate cyclin E-CDK2 activity, thereby influencing the progression from G1 to S phase and contributing to cellular differentiation processes.
p18: p18 is a cyclin-dependent kinase inhibitor (CDKI) that specifically targets cyclin E-CDK2 complexes, thereby preventing progression from the G1 phase to the S phase of the cell cycle.
p19: p19 is a cyclin-dependent kinase inhibitor (CDKI) that has been shown to interact with various cyclins, contributing to cell cycle regulation and potentially influencing cellular differentiation.
p21: p21 is a cyclin-dependent kinase inhibitor (CDKI) that functions to regulate cell cycle progression by inhibiting cyclin E-CDK2 complexes, thereby playing a critical role in maintaining cellular homeostasis and responding to DNA damage.
p27: p27 is a cyclin-dependent kinase inhibitor (CDKI) that plays a crucial role in cell cycle regulation by inhibiting cyclin D-CDK4/6 complexes, thus controlling the transition from the G1 phase to the S phase and contributing to cellular differentiation.
p57: p57 is a cyclin-dependent kinase inhibitor (CDKI) that helps regulate the cell cycle by inhibiting cyclin A-CDK2 complexes, thereby influencing both cell proliferation and differentiation.
Cip/Kip: This family of cyclin-dependent kinase inhibitors, which includes p21, p27, and p57, is essential for controlling cell cycle progression and ensuring proper cellular responses to environmental cues.
CDKIs are crucial in tumor suppression, as their loss or dysfunction can lead to uncontrolled cell proliferation and cancer development.
Cell Life Span and Turnover Rates
Blood B cells (mouse): 2-4 days
Stomach cells: 2-9 days
Trachea epithelial cells: 4-7 weeks
Blood Neutrophils: 1-5 days
Hematopoietic Stem Cells: 2 months
Sperm (male gametes): 2 months
Skin epidermal cells: 10-30 days
Central nervous system cells: 0.5-1 year (10% per year turnover)
Liver hepatocyte cells: 0.5-1 year
Chromosomes and Chromatids
Chromatid: One of two identical halves of a replicated chromosome.
Homologous chromosomes: Chromosomes that pair during meiosis.
Centromere: The region where two sister chromatids are joined.
Cell Cycle Checkpoints (LO2)
G1-S checkpoint: Checks if DNA is intact and ready to replicate.
G2-M checkpoint: Assesses if DNA replication is complete.
Metaphase checkpoint: Ensures chromosomes are aligned before separation.
P53 – The Guardian of the Genome (LO2)
A critical protein that acts as a transcription factor in response to DNA damage.
Functions include:
Arresting cell growth.
Activating DNA repair mechanisms.
Inducing apoptosis and maintaining genomic stability.
Meiosis (LO3)
Occurs only in germ cells to produce gametes (sperm and egg) to ensure the correct number of chromosomes.
Comprised of two rounds of cell division, resulting in four haploid cells.
Important for genetic diversity via processes such as recombination during Prophase I.
Meiosis Stages
Interphase I:
Single DNA strand duplication occurs, forming sister chromatids.
Meiosis I:
Two hapliod cells are produced from a single diploid cell.
Genetic diversity takes place due to recombination (crossover)
Prophase I:
Chromosome condesation
cross over and recombination between non-sister chromatids
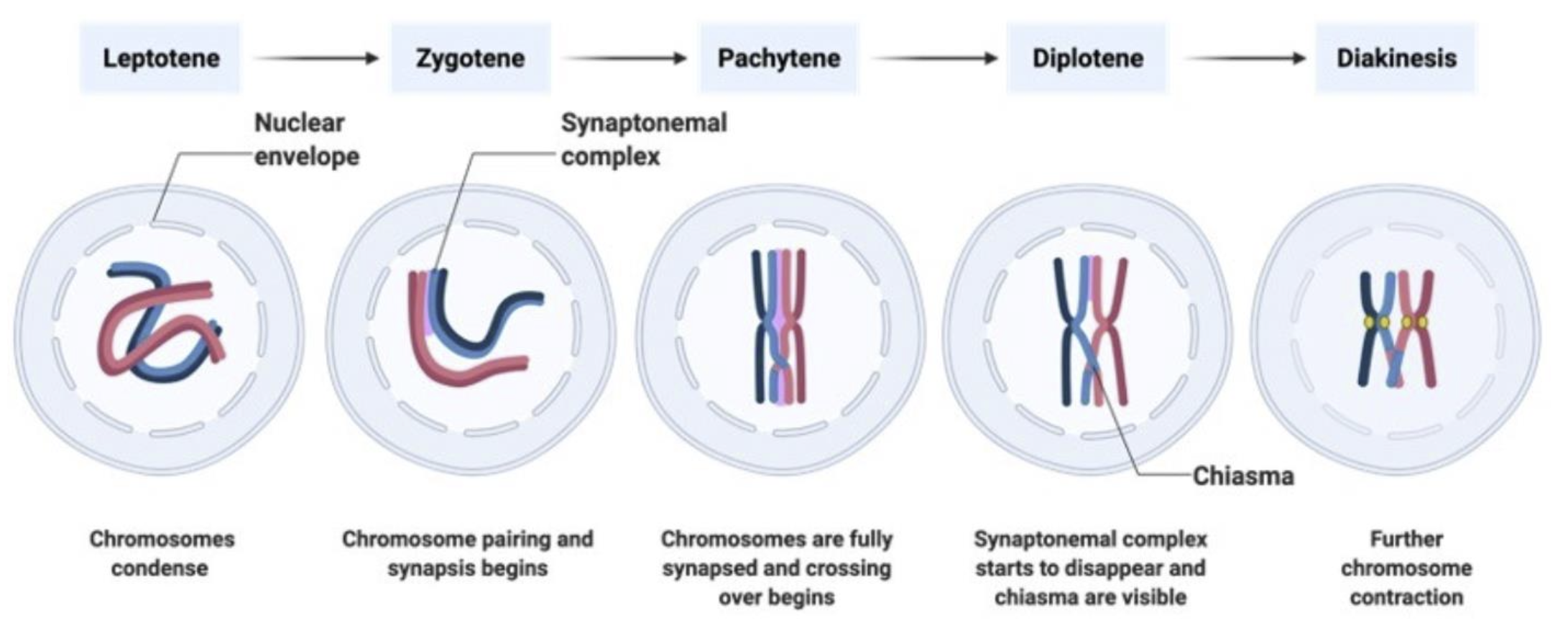
Leptonema (Stage I)
Leptonema is the first stage of Prophase I during meiosis.
In this phase, chromosomes begin to condense and become visible under a microscope.
Each chromosome consists of two sister chromatids that are joined at a centromere.
Leptonema is characterized by the pairing of homologous chromosomes in preparation for crossover and recombination processes that occur in later stages of Prophase I.
Zygonema (Stage II)
Zygonema is the second stage of Prophase I during meiosis.
During this phase, homologous chromosomes continue to condense and align with each other.
This is characterized by the formation of synaptonemal complexes, which facilitate the pairing of homologous chromosomes and promote genetic recombination (crossover).
Zygonema establishes the connections necessary for the exchange of genetic material between non-sister chromatids, which is crucial for genetic diversity in gametes.
Pachynema (Stage III)
All Chromosomes have aligned
Recombination occurs
exchange of material between the two non-sister chromatid creates diversity
Dyplonema (Stage IV)
Diplonema is the fourth stage of Prophase I during meiosis.
In this phase, the homologous chromosomes begin to separate but remain connected at crossover points called chiasmata.
The chromosomes continue to condense further, allowing for clearer visibility under a microscope.
This stage is crucial for ensuring genetic diversity through the exchange of genetic material that occurs during recombination in earlier stages.
Sister chromatids move away from each other
Sister chromatids are visible
Diakinesis (Stage V)
Diakinesis is the final stage of Prophase I during meiosis.
During this phase, the homologous chromosomes continue to condense and become maximally compacted.
The nuclear membrane begins to break down, and the spindle apparatus forms, preparing for the separation of homologous chromosomes.
Chiasmata (crossover points) are visible but begin to move towards the ends of the chromosomes, a process known as terminalization, which is crucial for subsequent chromosome segregation during meiosis.
Metaphase I
Spindles form betweem centrioles at opposite poles of the cell
Tetrads line up on the spindles on the metaphase plate
centromeres from homologous chromosomes on opposite sides
Random assortment introduces diversity
Anaphase I
Spindles pull homologous chromosomes apart
Each cell half has one of a pair of chromosomes (with crossed over material) and one sec chromosome
Telophase I
Nuclear membrane develops between each set of chromosomes
Cytoplasmic divisiom in males is equal whereas in females is unequal
Daughter cell with more cytoplasm becomes the egg
Meiosis II
mirrors mitosis, producing daughter cells that are haploid and diverse due to genetic recombination.
Prophase II
Nuclear envelope disntegrates
Each cell is haploid
Metaphase II
Chromosomes align at the metaphase plate, preparing for separation.
Anaphase II
Centromeres split
Sister chromatids pulled to opposite poles
Due to cross over form prophase I, the daughter cells will not be identical
Telophase II
begins with the reformation of the nuclear envelope around each set of chromosomes, followed by cytokinesis, resulting in four genetically diverse haploid cells.
At the end of meiosis
Males — Haploid cells with 22 chromosomes plus an X or Y chromosome
Females — Haploid cells with 22 chromosomes plus X chromosomes
Difference between Mitosis and Meiosis
What is differentiation
Cellular differentiation
Cell changes from one cell type to another
more specialised
from zygote to adult hood
changes in gene expression
Stem Cells and Differentiation
Stem Cells: Uncommitted cells capable of self-renewal and differentiation into various cell types.
Types of Stem Cells:
Totipotent: Can form all cell types plus placental tissue (e.g., zygote).
Pluripotent: Can develop into almost all cells in the body (e.g., embryonic stem cells).
Multipotent: Limited to differentiation into related cell types (adult stem cells).
Unipotent: Can only produce one cell type (germ line stem cell, epidermal stem cell
Induced Pluripotent Stem Cells (IPSCs): Created by reprogramming differentiated cells, holding potential for disease modelling and regenerative medicine.
Stem Cells in Regenerative Medicine (LO4, 5)
Stem cells are being studied to treat conditions such as:
Stroke
Alzheimer's disease
Parkinson's disease
Diabetes
Spinal cord injuries
And many others, demonstrating their potential in healing and restoring function in damaged tissues.