BIOL 2100 CH. 2 NOTES
Concept 2.1: Matter and Chemical Elements
Matter
Definition: Anything that takes up space and has mass.
Forms: Includes rocks, metals, oils, gases, and living organisms.
Elements and Compounds
Elements:
Cannot be broken down by chemical reactions.
Examples: Gold (Au), Copper (Cu), Carbon (C), Oxygen (O).
Each element has a unique symbol (e.g., Sodium = Na from Latin "natrium").
Compounds:
Substances made of two or more different elements in a fixed ratio.
Example:
Table salt (sodium chloride, NaCl) = Sodium (Na) + Chlorine (Cl).
Water (H₂O) = 2 Hydrogen (H) + 1 Oxygen (O).
Emergent Properties: Compounds exhibit characteristics different from their constituent elements.
The Elements of Life
Essential Elements:
Approximately 25 elements are essential for life.
Variation exists among organisms (e.g., humans need 25 elements; plants need fewer).
Major Elements in Living Matter:
Oxygen (O): 65%
Carbon (C): 18.5%
Hydrogen (H): 9.5%
Nitrogen (N): 3.3%
Other significant elements: Calcium (Ca), Phosphorus (P), Potassium (K), Sulfur (S).
Trace Elements: Required in minute quantities (e.g., Iron (Fe), Iodine (I)).
Example: Iodine is essential for thyroid hormone production; deficiency leads to goiter.
Major Elements:
Oxygen (O): 65%
Carbon (C): 18.5%
Hydrogen (H): 9.5%
Nitrogen (N): 3.3%
Others (Ca, P, K, S, Na, Cl, Mg): ~3.7%
Trace Elements: Less than 0.01% of body mass (e.g., Boron (B), Chromium (Cr), Iron (Fe), Zinc (Zn)).
Concept 2.2: An Element’s Properties Depend on the Structure of Its Atoms
Atoms and Elements
Atoms: Smallest unit of matter retaining element properties.
Element: Composed of a specific type of atom, symbolized by the element's abbreviation (e.g., C for carbon).
Subatomic Particles
Types:
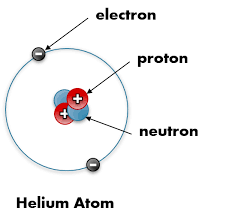
Protons: Positive charge, located in the nucleus.
Neutrons: Neutral charge, also in the nucleus.
Electrons: Negative charge, form a cloud around the nucleus.
Nucleus: Dense core of protons and neutrons; electrons are attracted to it due to opposite charges.
In an uncharged atom the number of protons is equal to the number of electrons, it’s electrically neutral
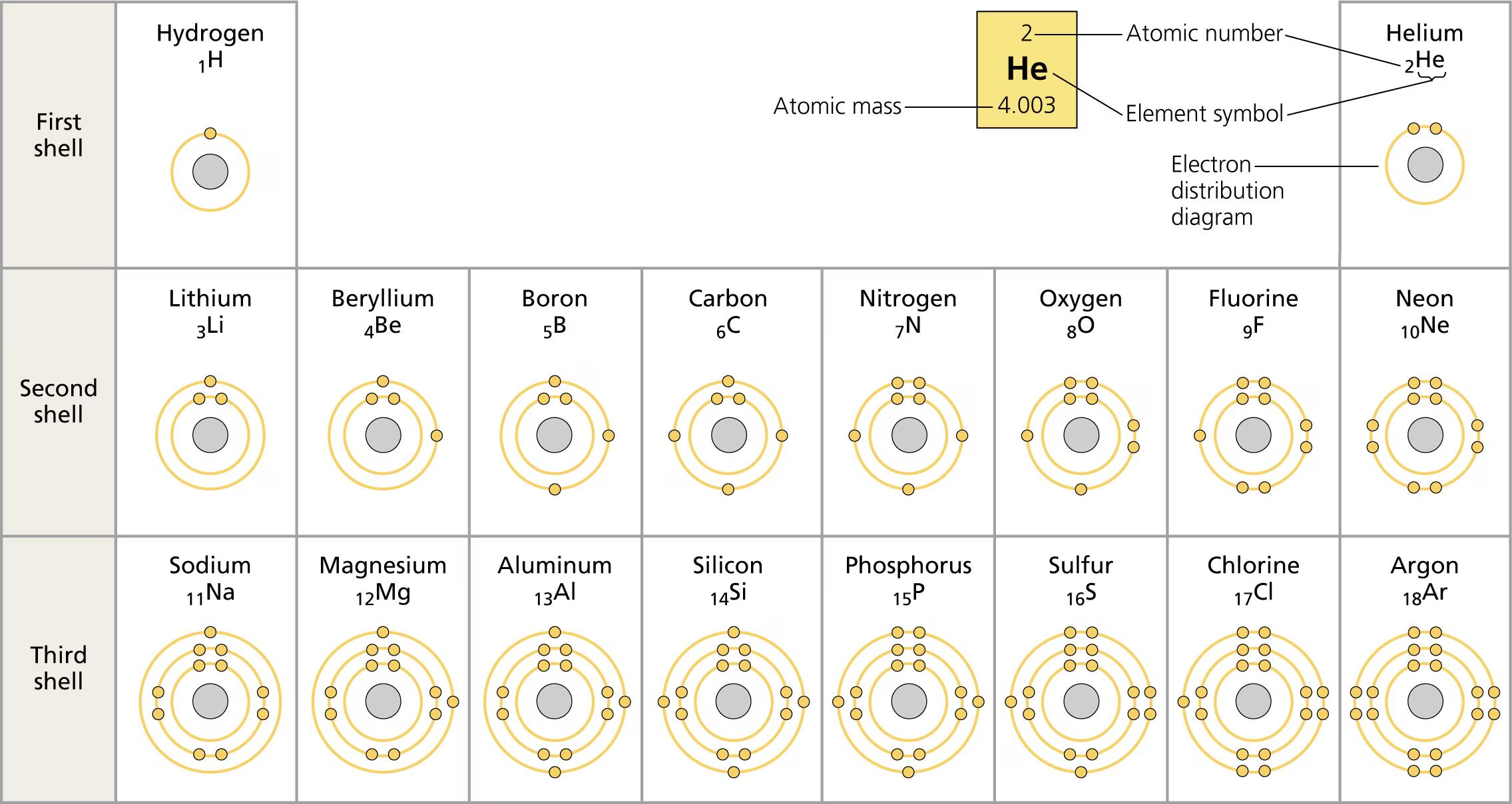
Atomic Number and Mass Number
Atomic Number (Z): Number of protons in the nucleus; indicates the element.
Mass Number (A): Total number of protons and neutrons.
Example: Helium (²He) has 2 protons and 2 neutrons.
Calculation of Neutrons: Neutrons = Mass Number - Atomic Number.

Isotopes
Definition: Atoms of the same element with different numbers of neutrons.
Example: Carbon has three isotopes (¹²C, ¹³C, ¹⁴C).
Stability: Stable isotopes do not decay; radioactive isotopes do, transforming into different elements.
Radioactive Isotopes
Uses:
Medical diagnostics (e.g., PET scans).
Tracers in biological research.
Radiometric Dating: Measures radioactive decay to date fossils and rocks.
Energy Levels of Electrons
Electron Shells: Electrons exist at fixed energy levels; further from the nucleus = higher potential energy.
Movement: Electrons can move between shells by absorbing or releasing energy.
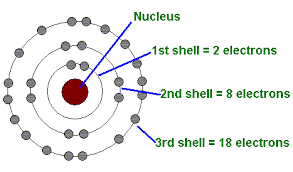
Electron Distribution and Chemical Properties
Valence Electrons: Electrons in the outermost shell determine chemical behavior.
Reactivity: Atoms with unpaired valence electrons are more reactive.
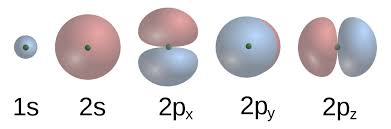
Electron Orbitals
Orbitals: Regions where electrons are likely found; different shapes (s, p, etc.) and orientations.
Shell Capacity:
1st shell: 2 electrons (1s)
2nd shell: 8 electrons (2s) (2 in each orbital)
Concept 2.3: Formation and Function of Molecules and Ionic Compounds
Chemical Bonding
Chemical Bonds: Atoms combine to form molecules and ionic compounds through interactions that complete their valence shells.
Types of Bonds:
Covalent Bonds: Sharing of valence electrons (e.g., H₂).
Ionic Bonds: Transfer of electrons resulting in oppositely charged ions (e.g., NaCl).
Covalent Bonds
Definition: A covalent bond involves the sharing of a pair of valence electrons between two atoms.
Will always be represented with a solid line between atoms
Equally shared electrons are non-polar covalent
Unequally shared electrons are polar covalent

Examples:
Hydrogen (H₂): Two H atoms share one pair of electrons.
Oxygen (O₂): Two O atoms share two pairs of electrons (double bond).
Water (H₂O): One O atom shares electrons with two H atoms.
Methane (CH₄): One C atom shares electrons with four H atoms.

Representations of Covalent Bonds
Molecular Formula: Indicates the number of atoms (e.g., H₂).
Electron Distribution Diagram: Shows shared electrons.
Lewis Dot Structure: Dots represent valence electrons.
Structural Formula: Lines represent shared pairs of electrons.
Space-Filling Model: Represents the actual shape of the molecule.
Ionic Bonds
Definition: Formed when one atom transfers an electron to another, creating cations and anions.
Will be represented by full oppsoite charges, not lines
Example: Sodium (Na) transfers an electron to Chlorine (Cl) to form Na⁺ and Cl⁻, resulting in NaCl.'

Properties: Ionic compounds form crystals and do not consist of individual molecules; their formula indicates the ratio of ions.
Weak Chemical Interactions
Importance: Weak interactions (e.g., hydrogen bonds, van der Waals interactions) are crucial for biological functions.
Hydrogen Bonds: Formed between a hydrogen atom covalently bonded to an electronegative atom and another electronegative atom.
Will always be represented by a dotted line btw. atoms
Van der Waals Interactions: Occur due to transient charges in molecules, allowing temporary adhesion. Can be very weak individually but together with multiple reactions it can be very powerful
Molecular Shape and Function
Shape Determination: Molecular shape is influenced by the arrangement of orbitals during covalent bonding.
It determines how biological molecules recognize and respond to one another

The single s and three p orbitals form four new hybrid orbitals shaped like identical teardrops extending from the region of the atomic nucleus
If we connect the larger ends of the teardrops with lines, we have the outline of a geometric shape called a tetrahedron,
For water molecules (H2O), two of the hybrid orbitals in the oxygen’s valence shell are shared with hydrogens.
The result is a molecule shaped roughly like a V, with its two covalent bonds at an angle of 104.5°.
The methane molecule (CH4) has the shape of a completed tetrahedron because all four hybrid orbitals of the carbon atom are shared with hydrogen atoms
Concept 2.4: Chemical Reactions and Bond Dynamics
Overview
Chemical Reactions: Processes that make and break chemical bonds, altering the composition of matter.
Example: Reaction of hydrogen (H₂) and oxygen (O₂) to form water (H₂O).
Bond Dynamics:
Breaks covalent bonds in H₂ and O₂.
Forms new bonds in H₂O.

Chemical Equations
Reactants: Starting materials (e.g., H₂ and O₂).
Products: Resulting materials (e.g., H₂O).
Arrow (→): Indicates conversion from reactants to products.
Coefficients: Indicate the number of molecules (e.g., 2H₂ means two molecules of hydrogen).
Conservation of Matter: Atoms are neither created nor destroyed; they are rearranged.
Photosynthesis
Importance: Fundamental biological process for food and oxygen production.
Raw Materials: Carbon dioxide (CO₂) and water (H₂O).
Process:
Sunlight powers the conversion of CO₂ and H₂O into glucose (C₆H₁₂O₆) and oxygen (O₂).
By-product: Oxygen gas released by aquatic plants (e.g., Elodea).
Energy Input: Sunlight is essential for the rearrangement of matter, it powers the conversion of carbon dioxide and water into glucose and oxygen

Chemical Reversibility
Reversible Reactions: Products can revert to reactants (e.g., ammonia formation and decomposition).
Dynamic Equilibrium:
Forward and reverse reactions occur at the same rate.
Concentrations of reactants and products stabilize at a specific ratio.
Equilibrium does not imply equal concentrations, just a stable ratio.
Factors Affecting Reaction Rates
Concentration of Reactants: Higher concentration increases collision frequency, enhancing reaction rates.
Accumulation of Products: Leads to increased reverse reaction frequency until equilibrium is reached.
Summary
Chemical reactions are essential for life, involving the rearrangement of atoms and the conservation of matter.
Understanding these processes lays the groundwork for studying the molecules vital to biological functions, such as water.
Chapter 2 Review Notes
Summary of Key Concepts
Concept 2.1: Matter and Chemical Elements
Elements: Cannot be broken down chemically; consist of pure forms.
Compounds: Combinations of two or more different elements in a fixed ratio.
Key Elements in Living Matter: Oxygen, carbon, hydrogen, nitrogen.
Concept 2.2: Atomic Structure and Properties
Atoms: Smallest unit of an element, composed of:
Nucleus: Contains protons (positive charge) and neutrons (neutral).
Electrons: Negative charge, form a cloud around the nucleus.
Atomic Number: Number of protons; defines the element.
Atomic Mass: Measured in daltons; roughly equal to the mass number (protons + neutrons).
Isotopes: Variants of an element with different neutron numbers; unstable isotopes exhibit radioactivity.
Electron Shells: Electrons occupy specific energy levels; incomplete outer shells indicate reactivity.
Orbitals: Three-dimensional spaces within shells that define electron distribution.

Concept 2.3: Chemical Bonding
Chemical Bonds: Form when atoms interact to complete valence shells.
Covalent Bonds: Formed by sharing electrons.
Single Covalent Bond: Sharing one pair of electrons (e.g., H₂).
Double Covalent Bond: Sharing two pairs of electrons (e.g., O₂).
Electronegativity: Attraction of an atom for electrons in a bond.
Nonpolar Covalent Bonds: Equal sharing of electrons.
Polar Covalent Bonds: Unequal sharing, leading to partial charges.
Ionic Bonds: Formed through the transfer of electrons, resulting in charged ions (e.g., NaCl).
Weak Interactions: Include hydrogen bonds and Van der Waals interactions, crucial for molecular shape and adhesion.
Molecular Shape: Determined by valence orbital positions; essential for biological recognition.


Concept 2.4: Chemical Reactions
Chemical Reactions: Transform reactants into products while conserving matter.
Reversibility: All reactions are theoretically reversible.
Chemical Equilibrium: Achieved when forward and reverse