2_Chemical_Context_of_Life_Lecture_Slides
Concept 2.1: Matter and Compounds
Matter: Anything that occupies space and has mass.
Composed of chemical elements and compounds.
Elements: Pure substances that cannot be broken down by chemical reactions.
Compounds: Substances formed from two or more elements in fixed ratios; possess emergent properties different from those of its elements.
The Elements of Life
Essential elements- about 20-25% of the 92 natural elements are essential for life.
Key Elements:
Major elements (96% of living matter): Carbon (C), Hydrogen (H), Oxygen (O), and Nitrogen (N).
Other essential elements (4%): Calcium (Ca), Phosphorus (P), Potassium (K), and Sulfur (S).
Trace elements: Needed in only minute quantities.
Elements in the Human Body
Percentage of Body Mass:
Oxygen (O): 65.0%
Carbon (C): 18.5%
Hydrogen (H): 9.5%
Nitrogen (N): 3.3%
Calcium (Ca): 1.5%
Phosphorus (P): 1.0%
Additional trace elements include Boron (B), Iodine (I), Iron (Fe), etc.
Concept 2.2: Atomic Structure
Atoms: Smallest unit of matter retaining the properties of an element.
Comprised of three types of subatomic particles:
Neutrons: No charge.
Protons: Positive charge.
Electrons: Negative charge, surrounding the nucleus in clouds.
Electrons form a “cloud” of negative charge around the nucleus.
Neutron and Proton mass are almost identical and are measured in daltons.
Electrons are not included when calculating the total mass of an atom.
Atomic Number: Number of protons in the nucleus.
Mass Number: Total of protons and neutrons (proton + neutron = mass number).
Isotopes: Two atoms of the same element with different numbers of neutrons. Some are radioactive.
Radioactive Tracers
Radioactive Isotopes: decay spontaneously, giving off particles and energy; often used as diagnostic tools in medicine.
Can be used to track atoms through metabolism, used with sophisticated imaging instruments like PET scanners which can help with monitoring growth of cancers in the body.
Radiometric Dating
A “parent” isotope decays into its “daughter” isotope at a fixed rate, expressed as the half-life of the isotope.
This technique allows scientists to determine the age of rocks and fossils, providing valuable information about the history of life on Earth.
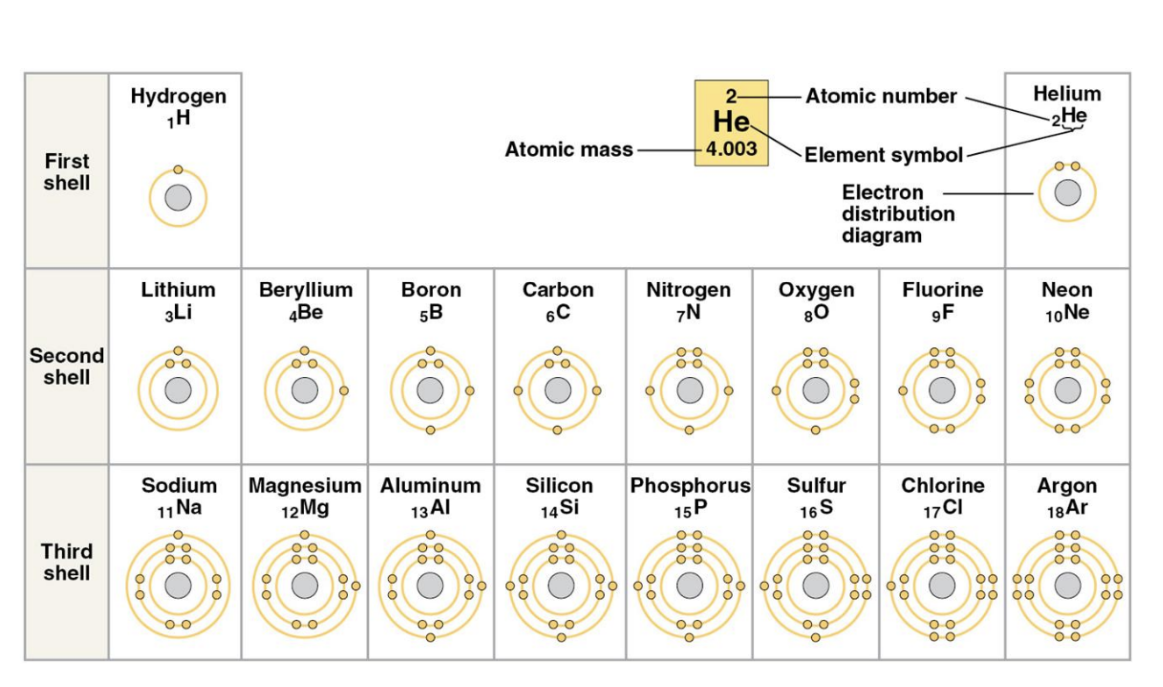
The Energy Levels of Electrons
Energy: the capacity to cause change.
Potential energy: the energy possessed by an object due to its position or state.
The electrons of an atom differ in their amounts of potential energy based on their difference from the nucleus; with electrons in higher energy levels being further away and thus having greater potential energy.
Electron shells: where electrons are found, each shell corresponding to a different potential energy level.
Valence electrons: the electrons in the outermost shell (valence shell) that are involved in chemical bonding and determine an atom's reactivity.
The chemical behavior of an atom is determined by the distribution of electrons in the electron shells.
Electron Orbitals:
Orbital: three-dimensional space where an electron is found 90% of the time.
Each electron shell consists of a specific number of orbitals; no more than 2 electrons can occupy a single orbital. These orbitals can be classified into different types, including s, p, d, and f orbitals, each with distinct shapes and energy levels.
Chemical Bonding; Covalent Bonds
Chemical Bonds: Attractive forces holding atoms together.
Covalent Bonds: Formed by sharing pairs of valence electrons; can form between atoms of the same or different elements.
Single Bond: Sharing one pair.
Double Bond: Sharing two pairs.
Structural Formulas: Representation of bonds. Example:
H—H represents a single bond, O == O represents a double bond.
Nonpolar covalent bond: Electrons are shared equally between atoms, resulting in no charge difference across the molecule.
Polar covalent bond: Electrons are shared unequally, leading to a partial positive charge on one atom and a partial negative charge on the other.
Ionic Bonds: Form when electrons are transferred from one atom to another, creating ions (cations and anions).
Cation: a positively charged ion.
Anion: a negatively charged ion.
Compound: a combination of two or more different elements.
Compounds formed by ionic bonds are called ionic compounds, or salts.
Electronegativity: an atom’s attraction for the electrons in a covalent bond; the more electronegative an atom is, the more strongly it pulls shared electrons toward itself.
Strength of Chemical Interactions
Weak interactions are crucial for biological functions.
Hydrogen Bonds: Form between a hydrogen atom covalently bonded to an electronegative atom (e.g., O or N).
Van der Waals Interactions: Weak attractions due to temporary charges in molecules.
Molecular Structure and Function
Molecule size and shape are critical for its function.
Hybridization: Orbitals can mix to form new shapes, influencing the properties of molecules.
Molecular shape determines interactions, such as how morphine mimics endorphins due to shape similarity.
Concept 2.4: Chemical Reactions
Chemical reactions involve the making and breaking of chemical bonds.
Reactants: Starting molecules of a chemical reaction.
Products: Resulting molecules of a chemical reaction.
Reactions can be reversible, with dynamic equilibrium when rates of forward and reverse reactions equalize.
Key Reactions in Biology
Photosynthesis is a vital chemical reaction converting CO2 and water into glucose and oxygen, powered by sunlight.
Summary of Key Concepts
Understanding the atomic structure aids in predicting chemical properties and behavior.
The type of bonding (covalent vs. ionic) influences compound formation and stability.
Weak interactions play a significant role in molecular biology and the structural integrity of biological macromolecules.