Knowt 7 - Chemical Bonding & Molecular Geometry
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Ionic Bonds
Strong electrostatic force of attraction between cations and anions in an ionic compound.
Covalent Bonds
Bond formed when electrons are shared between atoms.
Pure (Nonpolar) Covalent Bond
Equal Electrostatics share of electrons
Polar Covalent Bond
Unequal electrostatics share of electrons
Electronegativity
Tendency of an atom to attract electrons in a bond to itself.
Lewis Symbol
Illustrates the atoms valence electrons
Lewis Structures
Diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion.
Lone Pairs
Two (a pair of) valence electrons that are not used to form a covalent bond.
Single Bond
Bond in which a single pair of electrons is shared between two atoms.
Double Bond
Covalent bond in which two pairs of electrons are shared between two atoms.
Triple Bond
Bond in which three pairs of electrons are shared between two atoms.
Octet Rule
Fills up all 8 valence electrons
Free Radicals
Molecule that contains an odd number of electrons.
Hypervalent Molecules
Molecule containing at least one main group element that has more than eight electrons in its valence shell.
Formal Charge
A way to calculate the charge on an atom in a Lewis structure.
Resonance
When a molecule's structure is a mix of multiple possible electron arrangements.
Molecular Structure
The 3D arrangement of atoms in a molecule
Bond Length (Distance)
The shortest distance between the centers of two bonded atoms.
Bond Energy
The energy required to break a bond between two atoms in a gas.
Bond Angle
The angle formed by two bonds meeting at a shared atom.
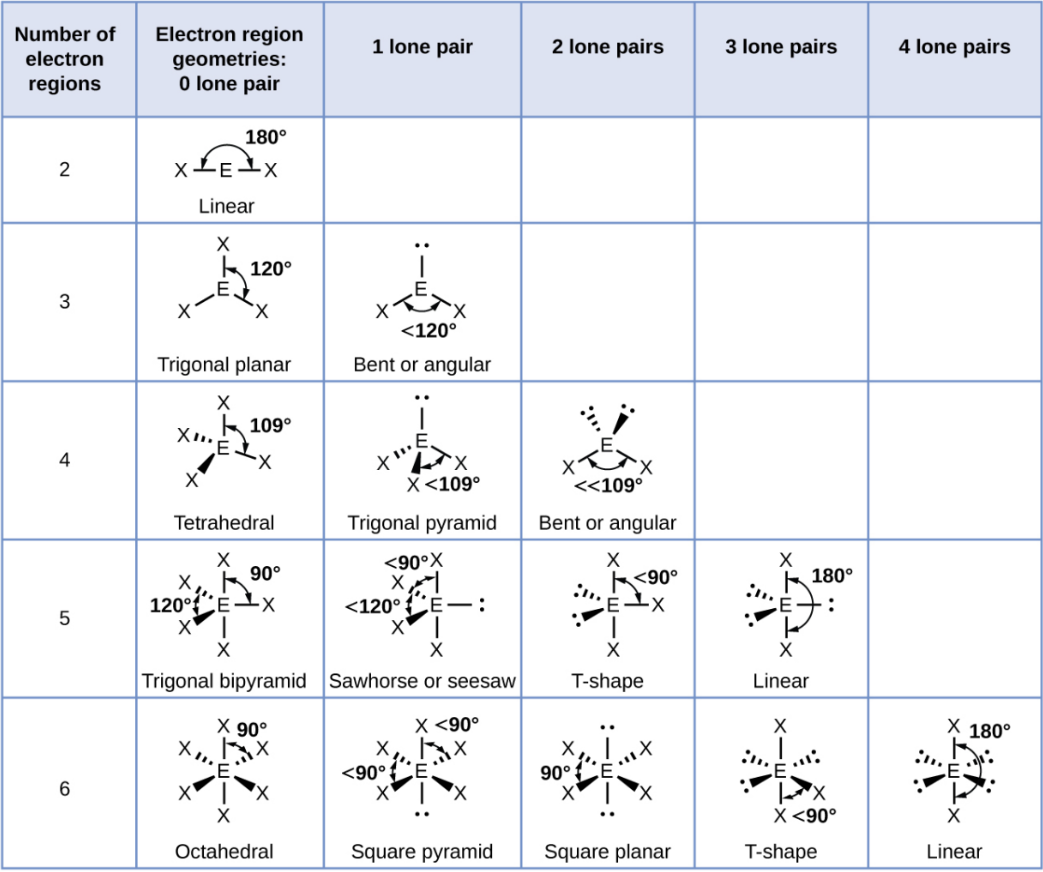
VSEPR Theory
Valence Shell Electron-Pair Repulsion
A theory predicting shapes by minimizing electron repulsions.
Linear
A straight-line arrangement with two groups opposite each other.
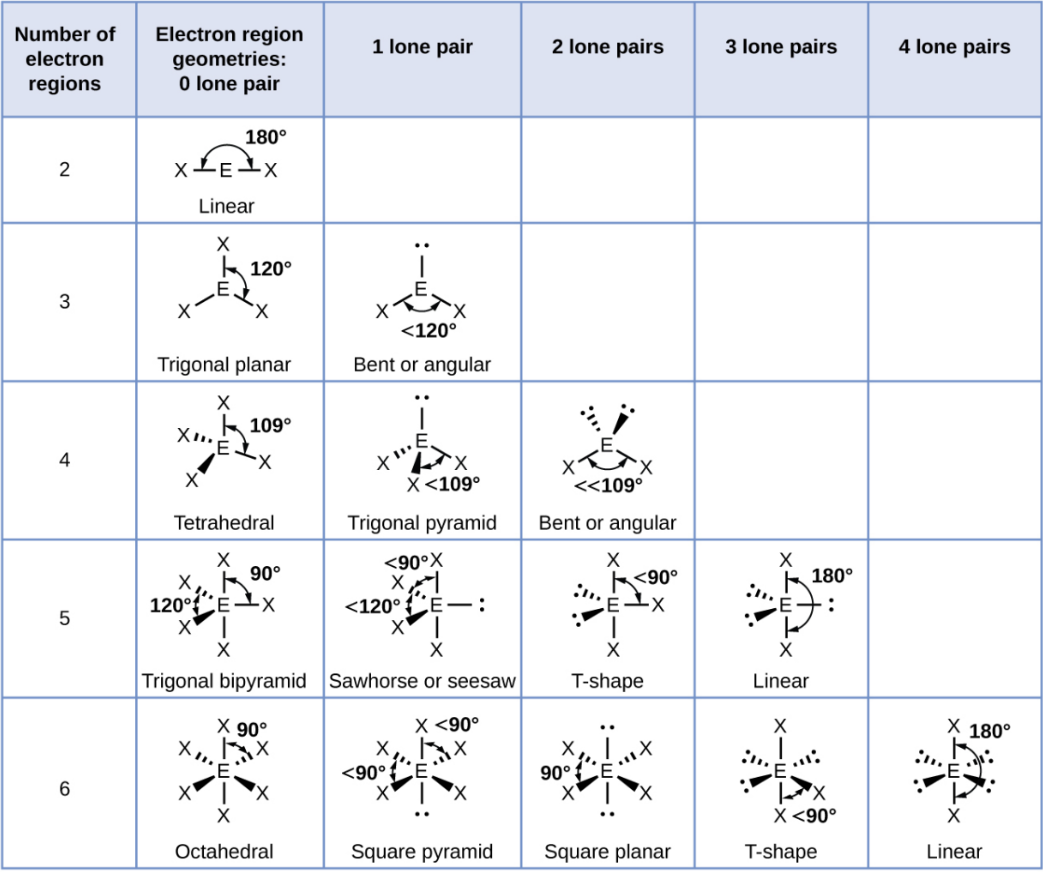
Trigonal Planar
A flat triangle shape with three groups at 120° angles.
Trigonal Planar - 1 Lone Pair
Bent or Angular
Tetrahedral
A shape with four groups at 109.5° angles around a central atoms
Tetrahedral - 1 Lone Pair
Trigonal Pyramid
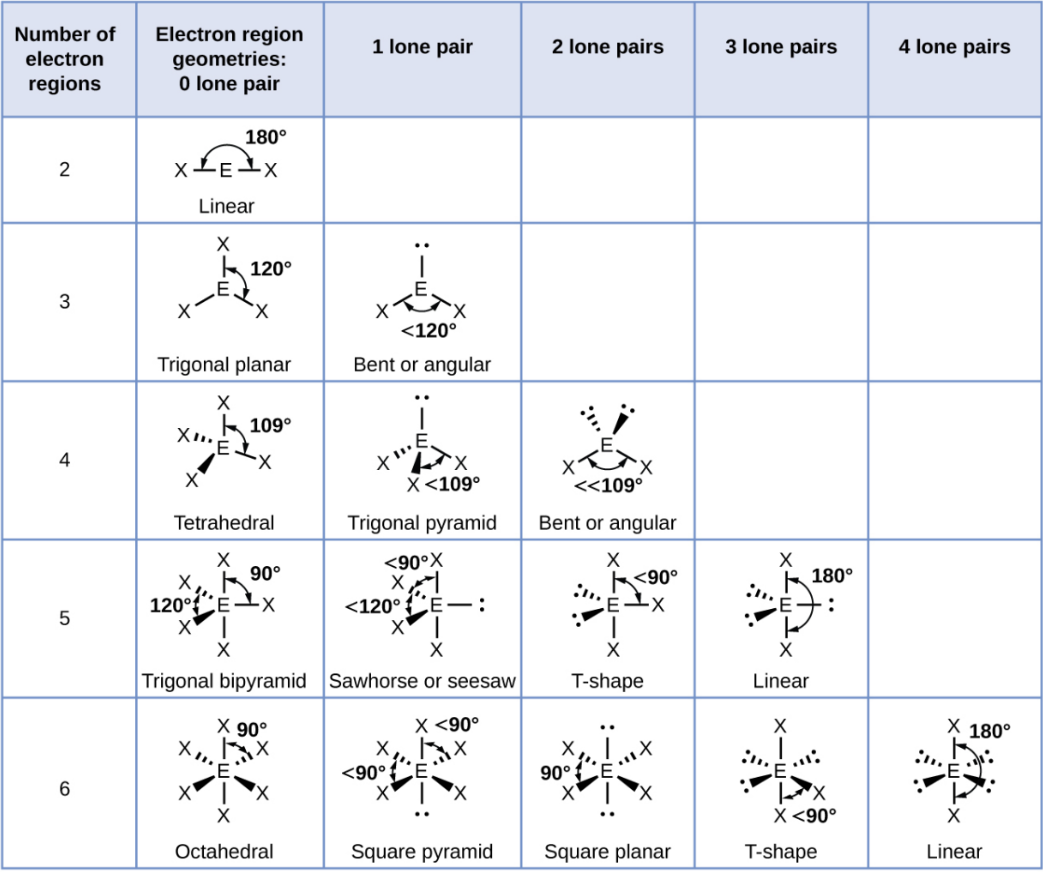
Tetrahedral - 2 Lone Pair
Bent or Angular
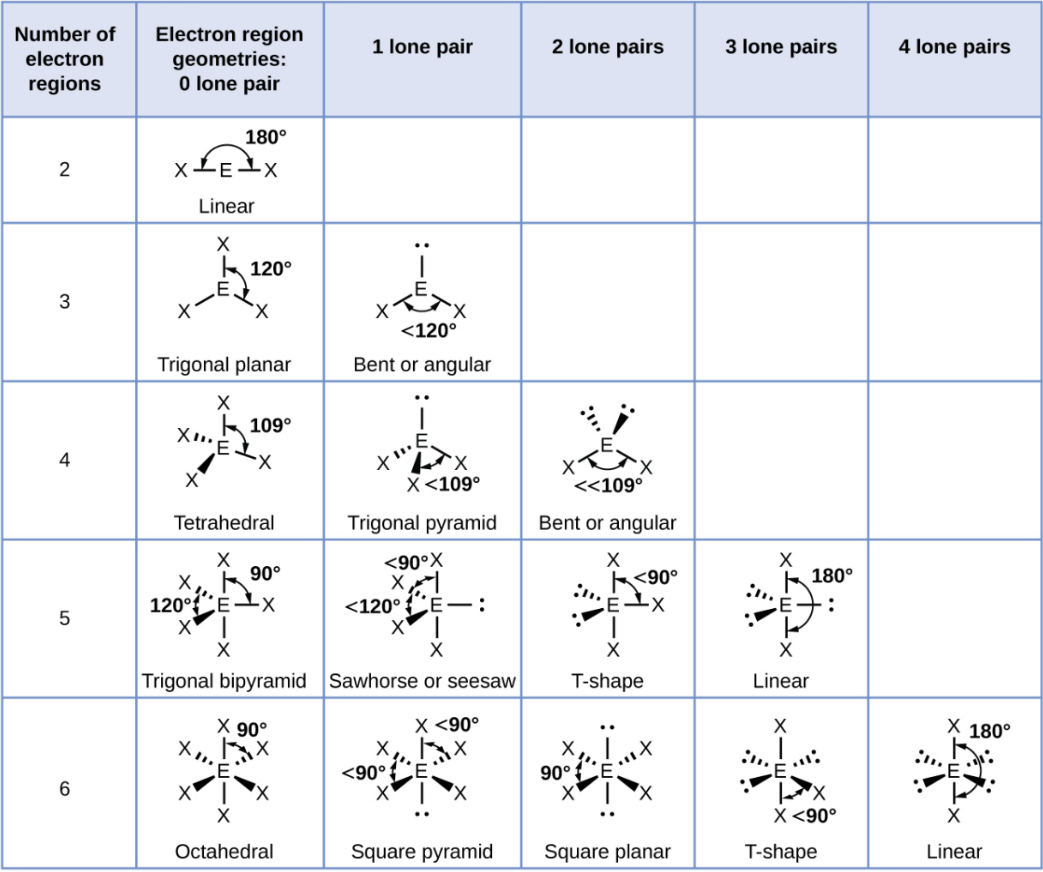
Trigonal Bipyramid
A shape with five groups: three in a triangle and two above/below.
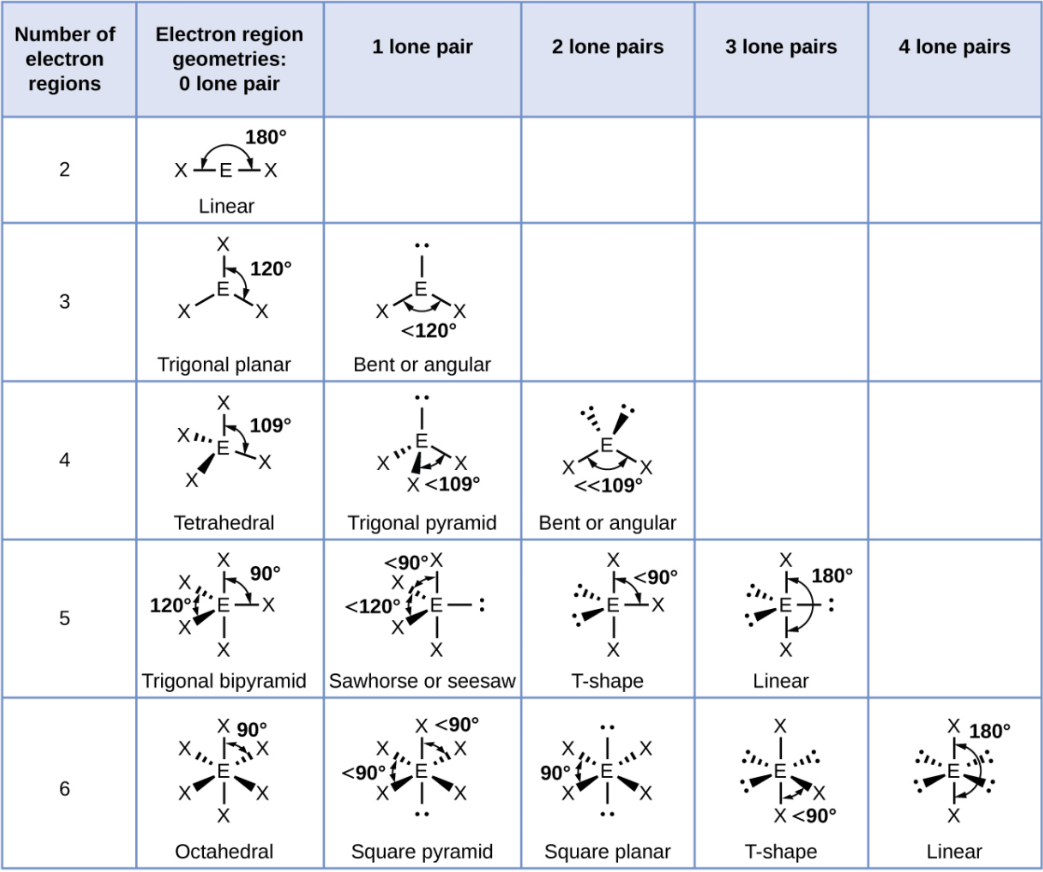
Trigonal Bipyramid - 1 Lone Pair
Sawhorse or Seesaw
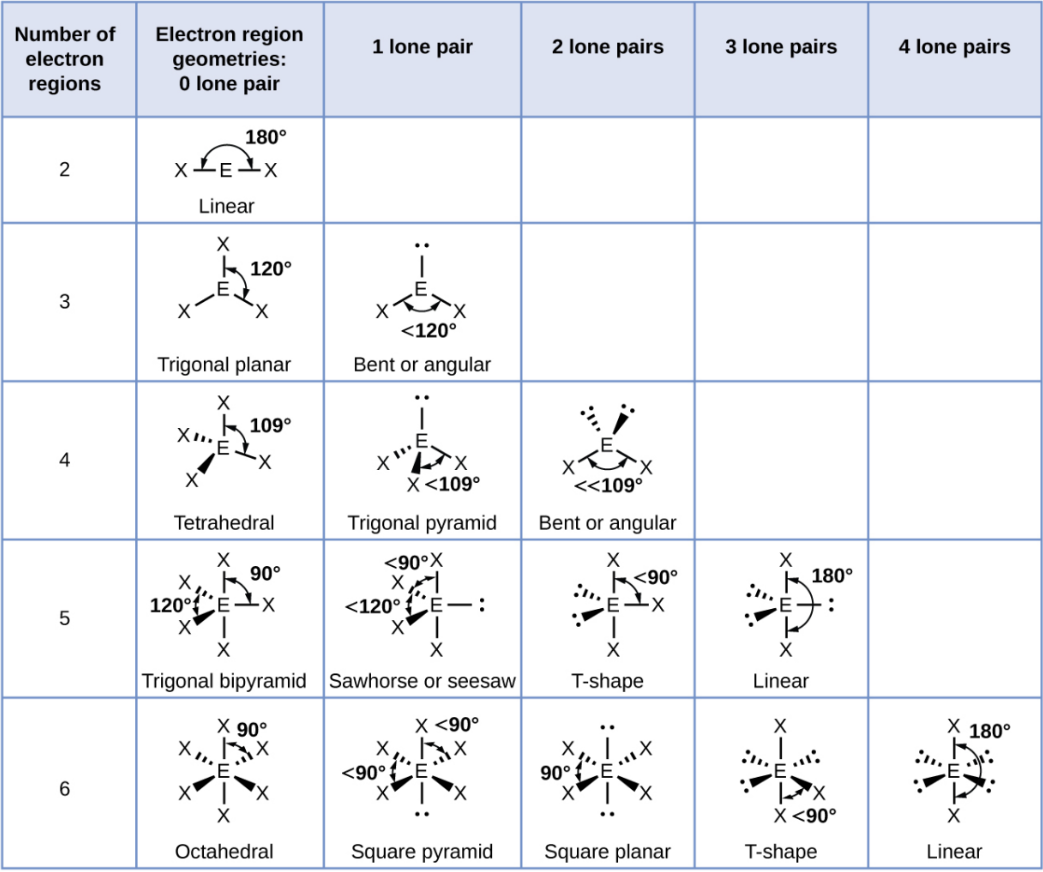
Trigonal Bipyramid - 2 Lone Pair
T-Shape
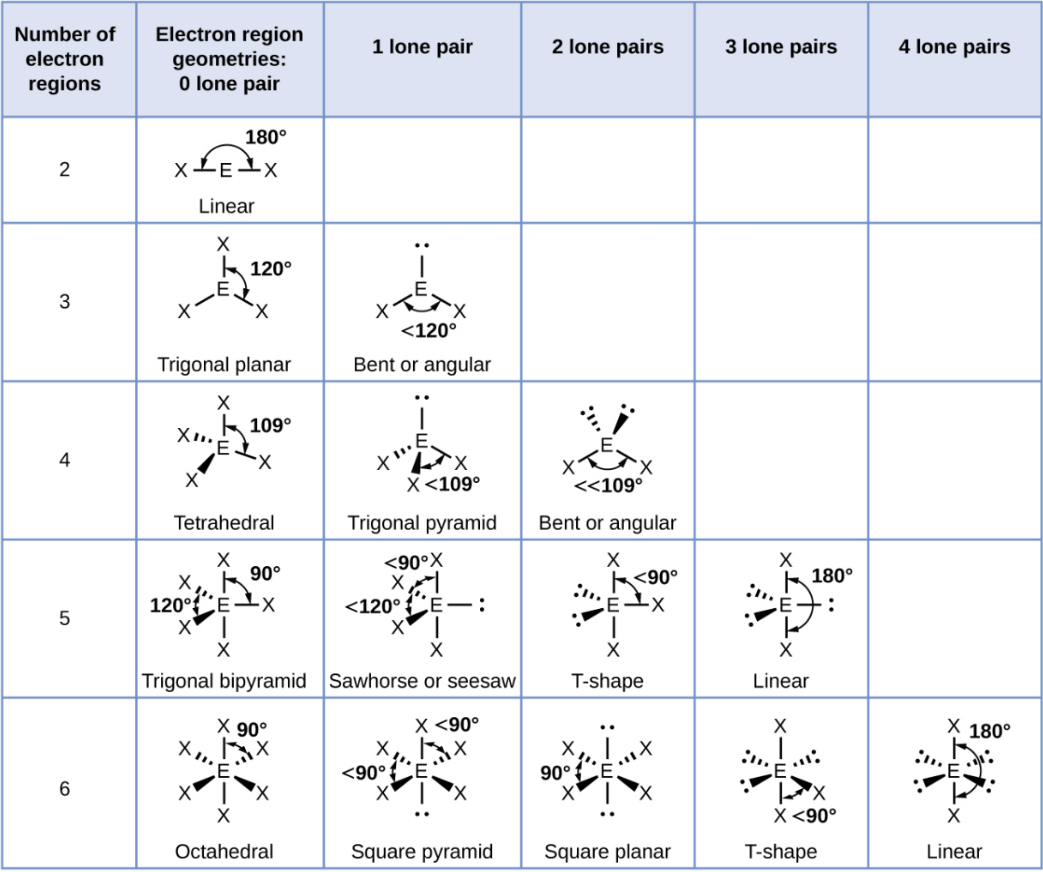
Trigonal Bipyramid - 3 Lone Pair
Linear
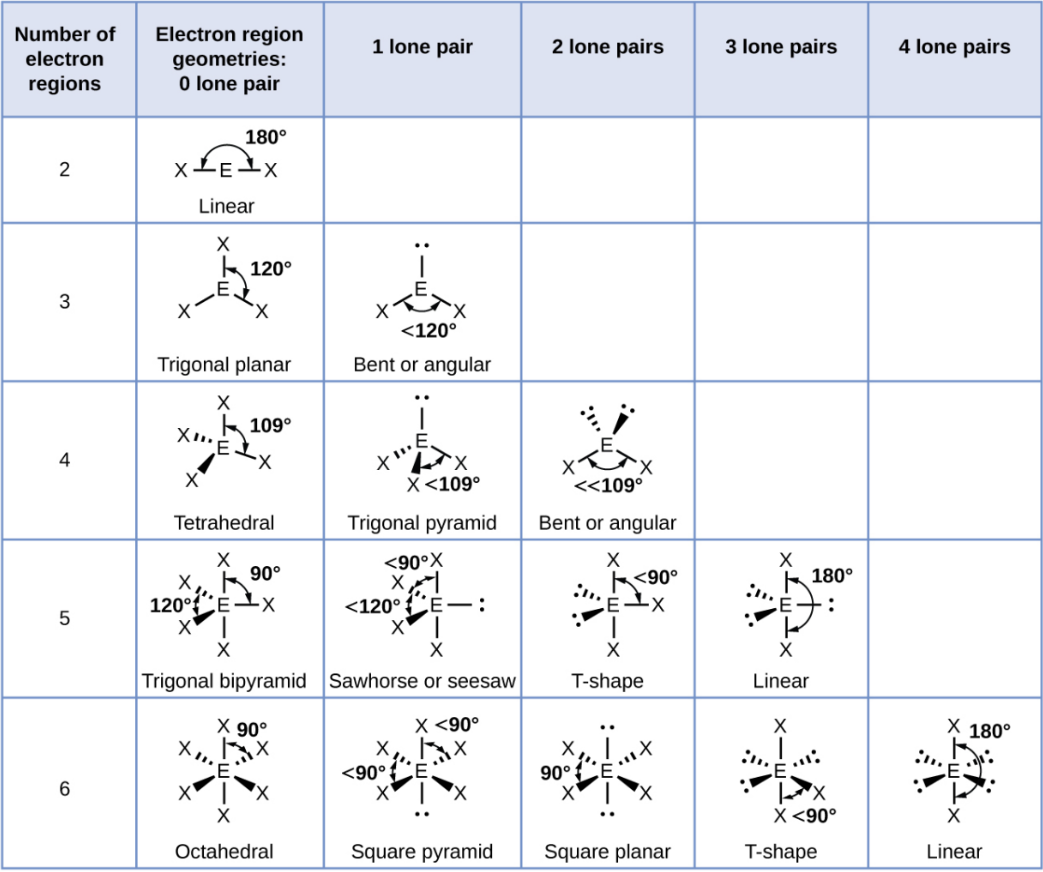
Octahedral
A shape with six groups around a central atom in a 3D square pyramid.
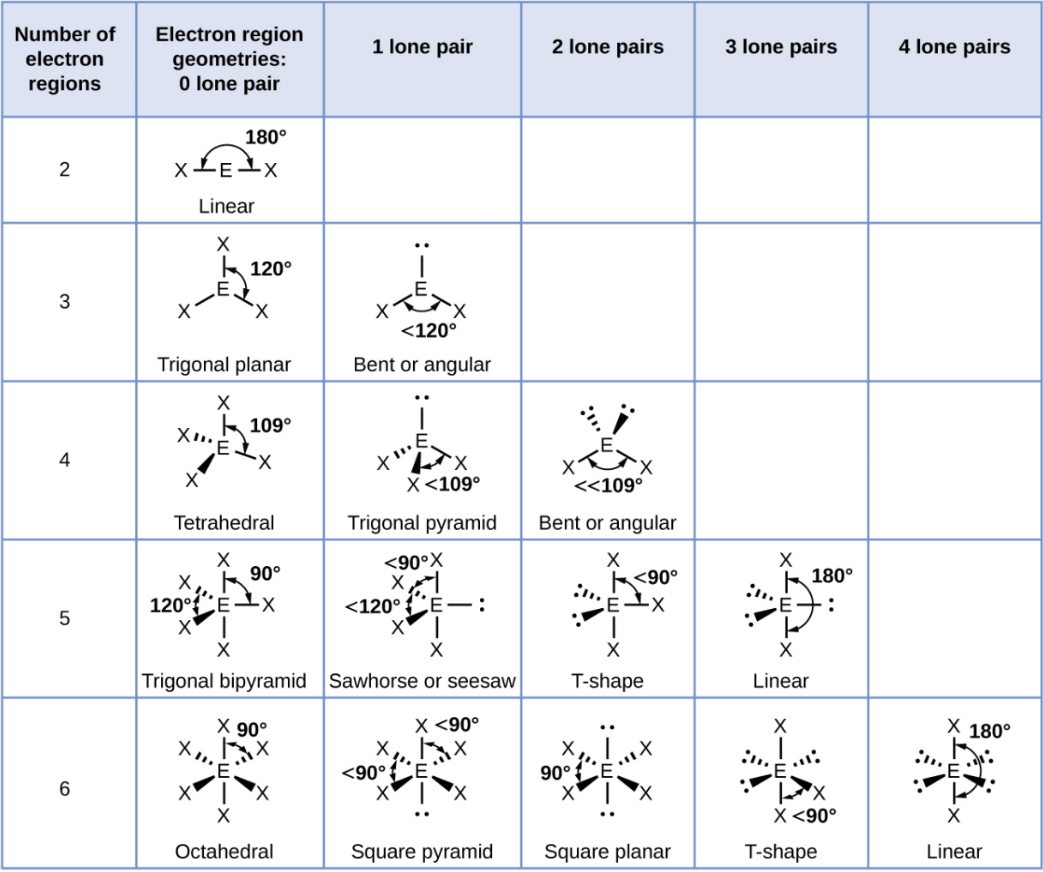
Octahedral - 1 Lone Pair
Square Pyramid
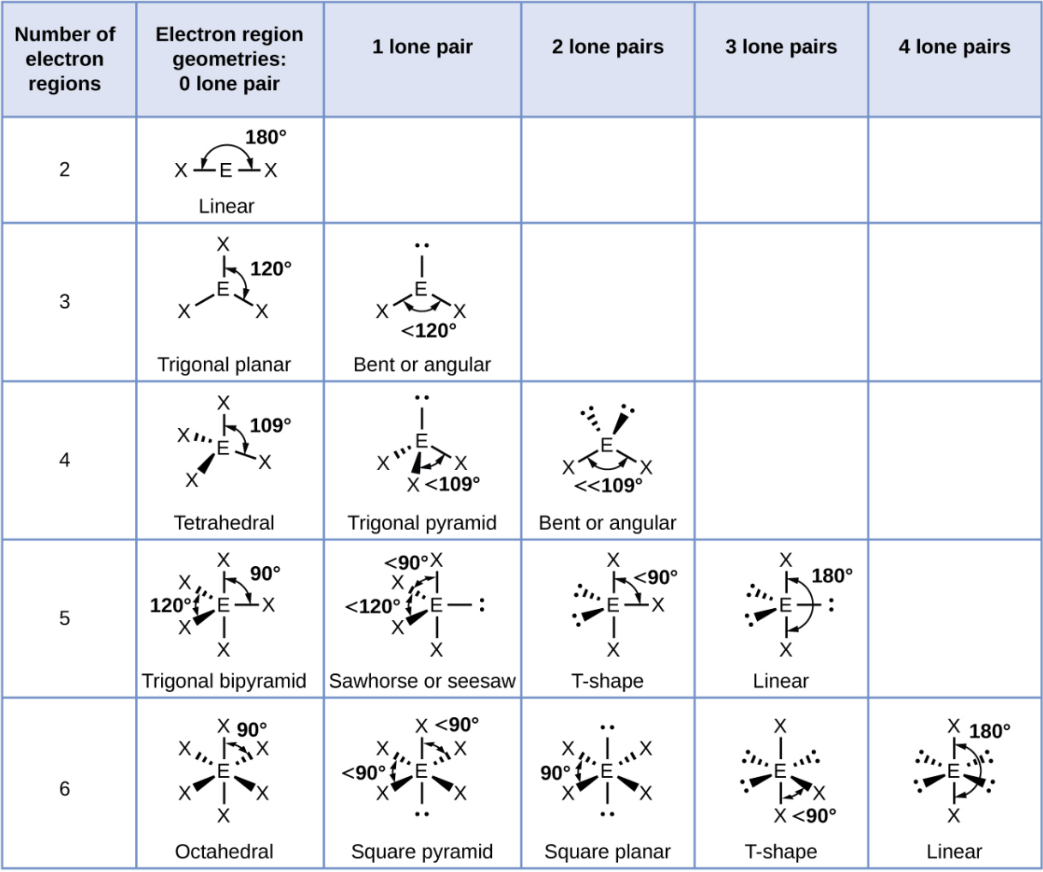
Octahedral - 2 Lone Pair
Square Planar
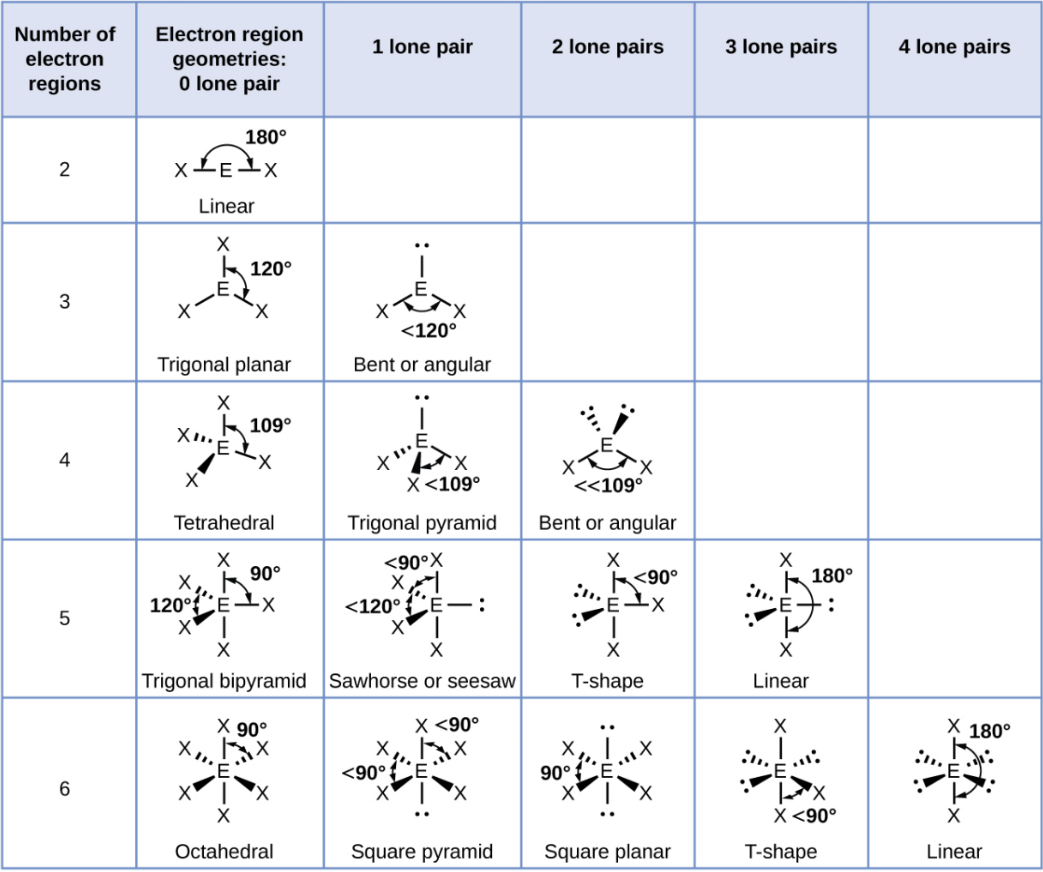
Octahedral - 3 Lone Pair
T-Shape
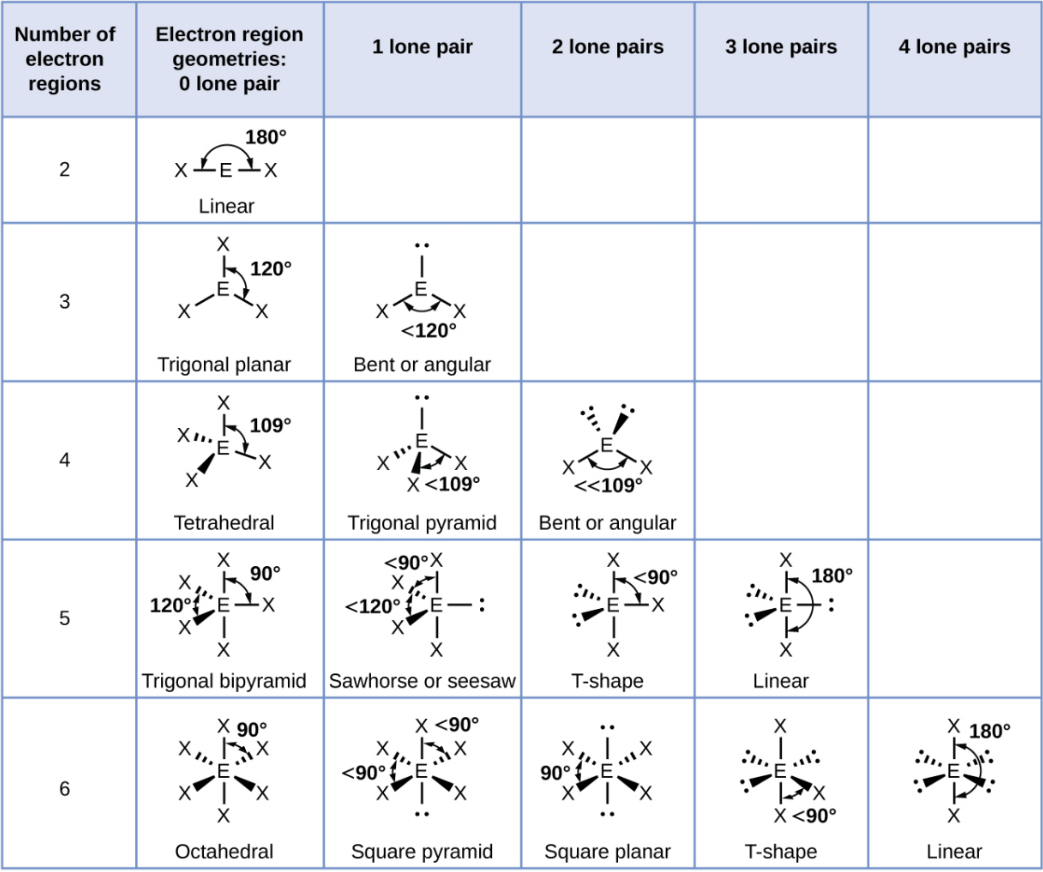
Octahedral - 4 Lone Pair
Linear
Electron - Pair Geometry
How electron groups are positioned around a central atom.
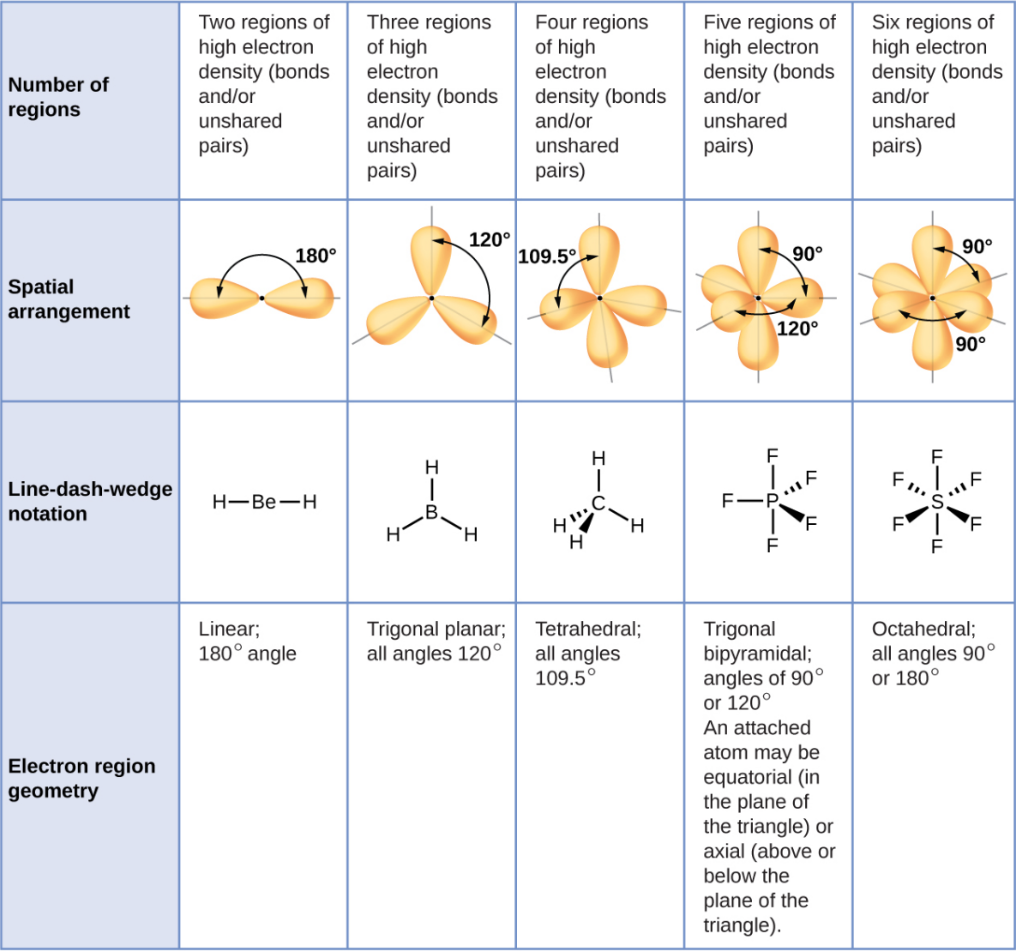
Axial Position
A position in trigonal bipyramidal shape at 180° from another.
Equatorial Position
One of three positions in trigonal bipyramidal shape at 120° angles.
Bond Dipole Moment
A measure of charge separation in a bond due to unequal electron sharing.
Vector
A quantity with both magnitude and direction.
Polar Molecule
A molecule with an uneven charge distribution.
Dipole Moment
The net separation of positive and negative charges in a molecule.
Formal Charge Equation
F.C = # valence shell electrons (free atom) - # lone pair electrons - ½ # bonding electrons
Bond Energy for a diatomic molecule - Equation Concept
XY (g) → X(g) + Y (g)
DX—Y = ∆H°
Enthalpy Change Equation
ΔH = ƩDbonds broken – ƩDbonds formed