LESSON 2: Stoichiometry
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Base
supports the bunsen burner and keeps it stable and upright on the laboratory table
Gas Inlet
the part through which gas usually enters the burner from the gas source
Collar
a moveable metal ring around the air holes that can be turned to open or close them
Air Holes
located at the bottom of the barrel ; they allow air to enter and mix with the gas
Combustion
the process of burning a substance (usually fuel) in the presence of oxygen
Complete Combustion
it produces carbon dioxide and water
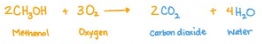
Complete Combustion
Incomplete Combustion
it produces carbon monoxide (toxic), soot, and water
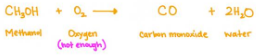
Incomplete Combustion
Electrolysis
chemical decomposition produced by passing an electric current through a liquid or solution containing ions ; in the case of water, it splits the H20 molecules into hydrogen gas (H2) and oxygen gas (O2)
Stoichiometry
a branch of chemistry that deals with the quantitative relationships between reactants and products
Empirical Formula
the simplest whole-number ratio of atoms in the compound
Molecular Formula
is a chemical formula that shows the number of atoms of each element in a molecule
Molar Mass
the mass (g) of one mole of a substance ; expressed in grams/mole or g/mol
6.02x10^23
1 mole = __________ particles of a substance
Theoretical Yield
is the maximum amount of product that can be produced from a given amount of reactant in ideal conditions
Actual Yield
is the amount of product actually produced when chemical reactions is carried out in an experiment