7.4 SN2 Nucleophilic Strength
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
what does nucleophilicity mean?
It refers to the rate at which a nucleophile attacks an electrophile- basically, how reactive the nucleophile is
How does nucleophilicity affect the SN2 reaction rate?
A strong nucleophile gives a fast SN2 reaction, while a weak nucleophile makes the reaction slow
Why is a strong nucleophile important for SN2 reactions?
Because SN2 is a one-step reaction, so it needs a strong nucleophile to attack quickly and make the reaction efficient and practical
What is one major factor that increases nucleophilicity?
The presence of a negative charge-negatively charged species are generally stronger nucleophiles
Which is the stronger nucleophile: hydroxide (HO-) or water (H2O)?
Hydroxide (HO—)- it has a negative charge, making it more reactive
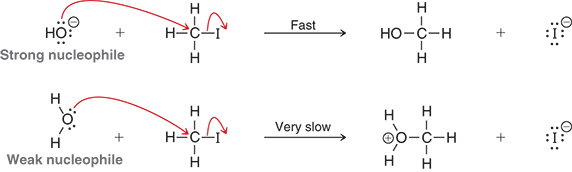
Why is water (H₂O) a weak nucleophile?
Because it is neutral (no negative charge), so it attacks electrophile less readily
How much more reactive is hydroxide than water in an SN2 reaction with methyl iodide?
Over a million times more reactive
What does this comparison (HO- vs H2O) show about nucleophilicity?
That charge matters- a negatively charged nucleophile reacts much faster than a neutral one in SN2 reactions
What happens to an SN2 reaction if the nucleophile is weak?
The reaction becomes too slow to be practically useful
What is polarizability?
The ability of an atom’s electrons to shift or spread out in response to external influences
How is polarizability related to nucleophilicity?
More polarizable atoms (larger atoms with more distant electrons) are stronger nucleophiles
What makes an atom more polarizable?
Having a larger size and electrons farther from the nucleus that can easily move
Why are sulfur-based ions strong nucleophiles?
Sulfur atoms are large and highly polarizabl, making ions like HS- and RS- very strong nucleophiles
Which halogen is a good example of highly polarizable and strong nucleophile?
Iodide (I-)- it is large and its electrons are far from the nucleus, so it’s very polarizable and reactive in SN2 reactions
Complete the phrase “When it comes to nucleophilicity, ____ is better.”
Bigger- larger, more polarizable atoms make stronger nucleophiles
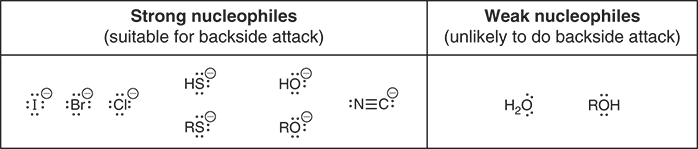
Why is fluoride (F-) not always listed as a strong nucleophile?
Because its strength depends on the solvent- it can act as either a weak or strong nucleophile
What does fluoride’s behavior illustrate about SN2 reactions
That the solvent has a major effect on how fast an SN2 reaction occurs
In what kind of solvent is fluoride a weak nucleophile?
In polar protic solvents (like water or alcohols), where it forms hydrogen bonds and get trapped
In what kind of solvent is fluoride a strong nucleophile?
In polar aprotic solvents (like acetone or DMSO), where it’s free to attack
What does “SN2” stand for?
Substitution Nucleophilic Bimolecular-both the nucleophile and substrate affect the rate
What phrase can help you remember how SN2 works?
“Backside attack”- the nucleophile attacks from the side opposite the leaving group
Why does SN2 cause inversion of configuration?
Because the nucleophile attacks from the backside, flipping the molecule like an umbrella turning inside out
What two main factors control SN2 rate?
Strength of the nucleophile and steric
What kind of mechanism is an SN2 reaction?
A one-step mechanism involving backside attack — the nucleophile attacks as the leaving group leaves.
What does “SN2” stand for?
Substitution Nucleophilic Bimolecular — both the nucleophile and substrate affect the rate.
What order of kinetics does SN2 follow?
Second-order kinetics — the rate depends on both the nucleophile and the substrate.
What kind of leaving group is needed for an SN2 reaction?
A good leaving group with little steric hindrance — methyl and primary alkyl halides work best.
Which alkyl halides are fastest in SN2 reactions?
Methyl and primary alkyl halides — they have the least steric hindrance.
Which alkyl halides do not undergo SN2 reactions?
ertiary and neopentyl alkyl halides — they are too crowded for backside attack.
What kind of nucleophile is required for SN2 reactions?
A strong nucleophile — to efficiently attack the electrophilic carbon.
What happens to stereochemistry in an SN2 reaction?
It undergoes inversion of configuration — like an umbrella flipping inside out.