Shapes and IMFS
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
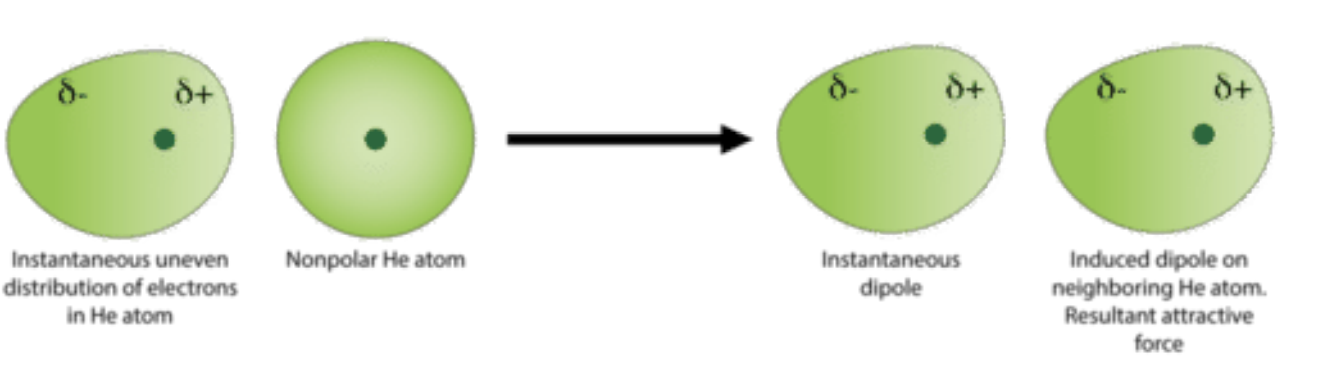
Van der Waals forces / Induced dipole-dipole
Weakest type of IMF
Act as an induced dipole between molecules
Substances w/ simple molecular structure consist of covalently bonded molecules held together with weak Vdw forces
Strength: depends on Mr of molecule and its shape (larger Mr - stronger IMFs) (straight chain > branched chain as they can pack closer together: reduces distance which force acts making it stronger)
Act between alkane chains
Effected by chain length and presence of branching
Permanent Diole
Type of IMF which acts between molecules with a POLAR BOND
The positive and negative dipoles on adjacent molecules attract each other and hold molecules together in a lattice-like structure
Stronger than vdW forces - more energy needed to overcome them, so higher mpts and bpts
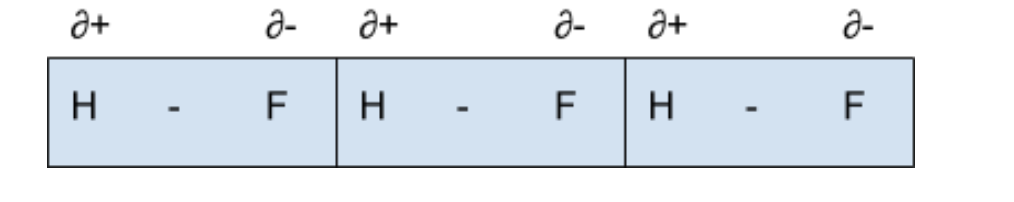
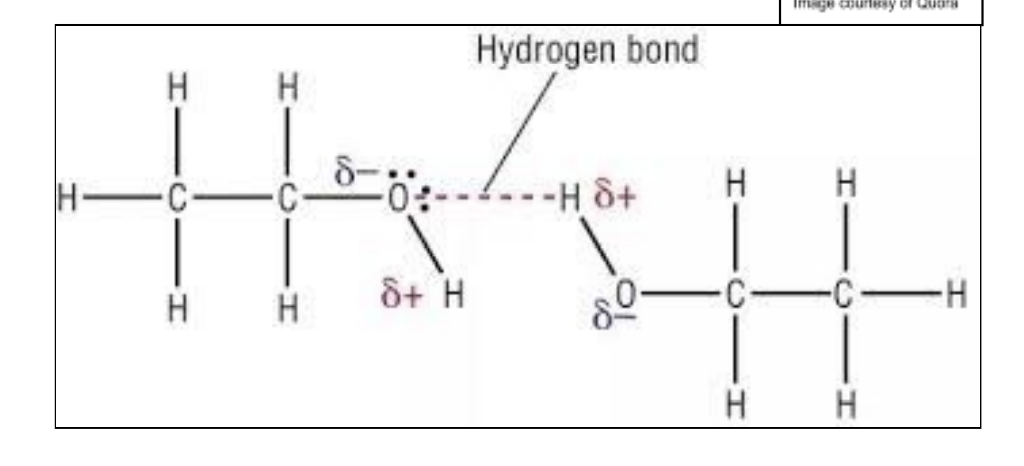
Hydrogen Bonding
Strongest type of IMFs
Act only between hydrogen and 3 most EN atoms: N, F and O
Lone pair of e- on these atoms forms bond with positive dipole on hydrogen atom
Much higher mpts and bpts than atoms without H bonding
Type of IMF heavily influences physical properties
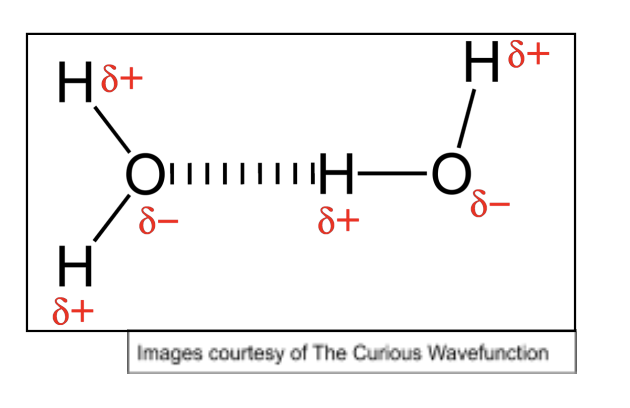
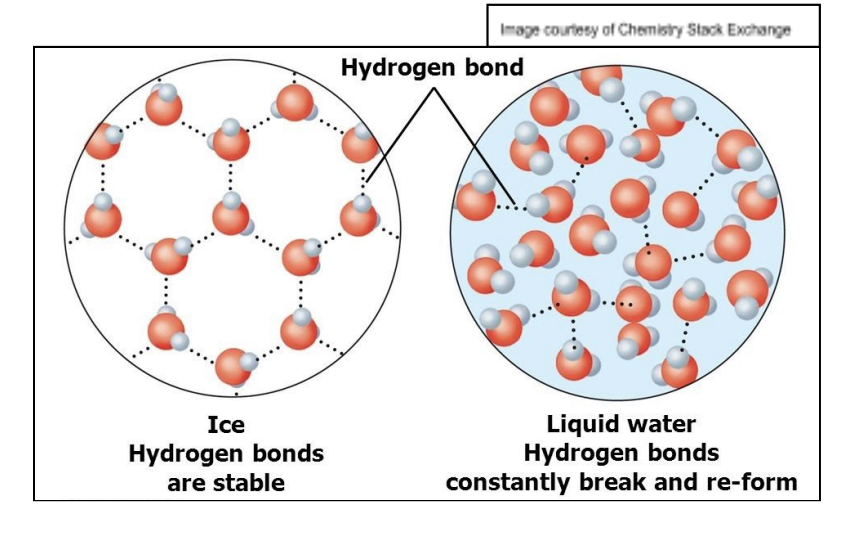
Water’s unusual properties
Simple molecule with unusually high mpts and bpts for size of molecule due to Hydrogen bonds
Hydrogen bonds also result in ice having much lower density than liquid water, as they hold the molecules in a rigid structure w/ lots of air gaps
Properties of alcohols due to Hydrogen bonding
Much higher bpts than alkanes w/ similar Mr value: one pair of electrons on Oxygen atom in alcohol is able to form hydrogen bonds witha hydrogen bonded to oxygen on a neighbouring alcohol molecule
Alcohols and water good solvents for compounds that can form H bonds in solution
Poor for dissolving of some polar molecules (halogenoalkanes) which cannot form H bonds
H- bonds in DNA
AT base pair held together by two H-bonds
GC base pair held together by three H-bonds
Determining molecule shapes
Find no. of e- pairs
Determine how many pairs are bonding / lone
Bonding pairs indicate basic shape, lone pairs indicate additional repulsion
Lone pair repulsion
Bond angle reduced by 2.5º
Linear
2 bonding e- pairs
0 lone pairs
180º bond angle

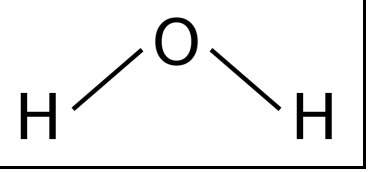
Non-Linear
2 Bonding pairs
2 lone pairs
104.5º angle
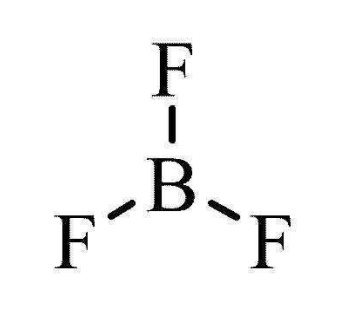
Trigonal Planar
3 bonding pairs
0 lone pairs
120º bond angle
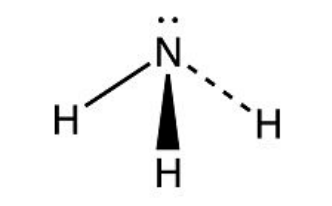
Triangular Pyramid
3 bonding pairs
1 lone pair
107º bond angle
Tetrahedral
4 bonding pairs
0 lone pairs
109.5º bond angle
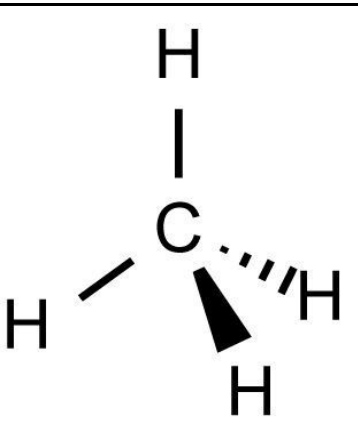
Trigonal Bipyramid
5 bonding pairs
0 lone pairs
180º and 120º bond angle
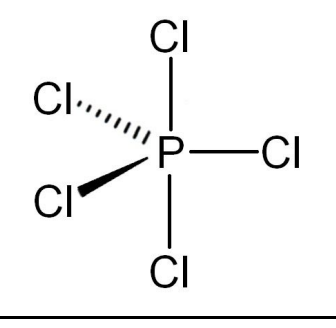
Octahedral
6 bonding pairs
0 lone pairs
90º bond angle
Electronegativity
Ability of an atom to attract the bonding electrons in a covalent bond towards itself
Depends on size and nuclear charge
Increases along period (AR decreases and charge density increases)
Decreases down group (shielding increases and AR increases → charge density decreases)
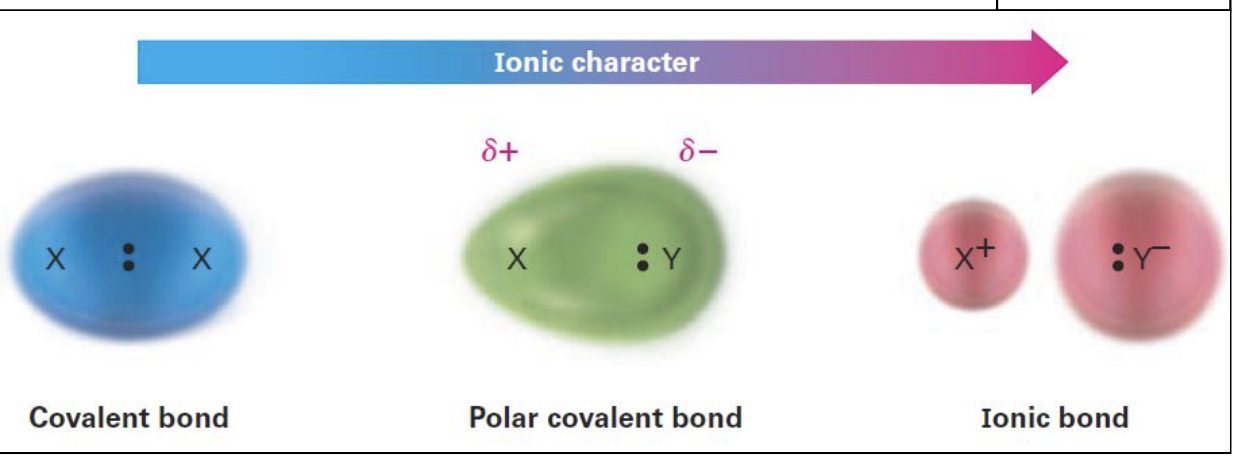
Ionic Character
Covalent bond
Polar covalent bond
Ionic bond
Can be permanent or induced
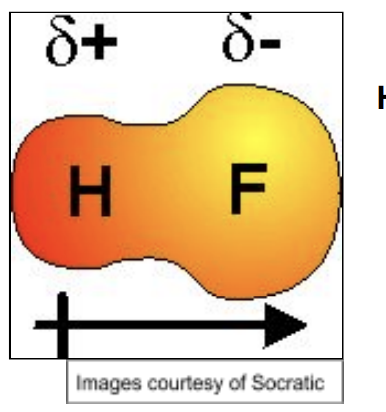
Permanent Dipole
Two bonded atoms w/ different electronegativities
More EN atom draws more negative charge towards itself and away from other atom
Polar molecule requires polar bonds that do not cancel due to direction
CO2 - contains polar bonds but symmetrical SO non-polar
Induced Dipole
Can form when eletron orbitals around a molecule are influenced by distributions of electrons on another particle