Bioinorganic chemistry
1/44
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
What are proteins containing metal ions called?
metalloproteins
What is the pattern of atomic radii?
increases down a group, decreases from left to right accross a period
What is the pattern of ionization energy for the 1st and 2nd groups and the 1st period?
1st ionization energy decreases down group 1 and 2, accross the 1st period it increases left to right
What is the pattern of electron affinity?
elements towards the top right of the table have the highest
What is the pattern of electronegativity?
the top right of the perodic table is most electronegative
What is polarizability?
the ease with which an atom or ion can be distorted by an electric field
What makes an atom, acid or base hard?
they have high ionization energies, they are less polarizable and form bonds with more ‘ionic character’
What makes an atom, acid or base soft?
they have low ionization energy and electron affinity, they are more polarizable and form bonds with more ‘covelent character’
What are the biological roles performed by metals?
structural, catalytic, redox, and some other ones
What are the factors that influence the biological role of the metal?
valency, ionic radius, polarizability, hydration energy, radius of the hydrated ion
What is hydration energy?
ease with which water molecules can be removed from the metal ion
What does the Lewis Acid and Bases theory state?
hard acids prefer hard bases, soft acids prefer soft bases
How does molecular shape of metal coordination impact the biological role of the metal?
each metal has a shape they have a preferance for which determines their biological funtion
What are three most common coordination geometries?
4,6 and 5
What are the geometries for a coordiation number of 4?
square planar, tetrahedral

What are the geometries for a coordiation number of 6?
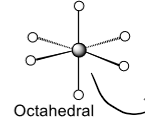
octohedral
Monodentate ligand
only one point of attachment to the metal
Polydentate ligand
more than one point of attachment to the metal (these are very important in biology)
Macrocyclic ligands
a cyclic molecule with at least 9 atoms containing at least 3 donor atoms (lewis base centers) , they are thermodynamically and kinetically more stable than non-cylic ligands
What is the chelate effect?
complexes with polydentate ligands will be more stable than complexes with similar monodendate ligands this is due to the favourable entropic factor accompanying the release of nonchelating ligands from the metal ion, stability increases with the number of donor atoms in the ring.
What is the porphyrin unit?
haem unit, it is a stable arrangement as there is only one ligand, there are 2 remaining sites on the Fe for oxygen to bind

What is the clinical significance of chelation?
as metal ions are widely distributed throughout the body, and a range of drugs can behave as chelating ligands, chelation changes physical and chemical characteristics of both componanys
What is the mode of action of tetracyclines?
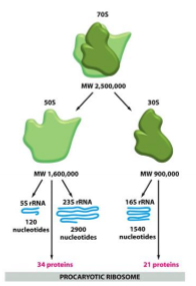
bacteriostatic agents that target the 30S subunit of bacterial ribosomes - selective binding, they block the binding of aminoacyl tRNA in the A-site which is one of the binding sites in the channel, there are Mg²⁺ ions, the bottom face of tetracyclines are full of oxygen atoms which are strong lewis bases and engage in metal chetalation
Bacterialcidal agent
Kills the bacteria, immune system isn’t involved
Bacteriostatic agents
stop bacteria growing and reproducing, the immune system kills the bacteria
How are tetracyclines affected by drug interactions?
absorption is affected by chelation with metal ions as most of the range of metal chelates are insoluble
How does tetracyclines cause discouloration?
can permently discoulor teeth for children under as the drug collates with the calcium in the tooth, if taken while pregnant or breast feeding tetracycline ends up in baby bones which effects skeletal growth
How do metal ions affect conjugate bases?
they stablise them and the pKa of the parent acid is lowered, the conjugate base becomes a weaker base as it is stabalised
What are carbonic anhydrases?
enzymes found in most tissue types that are involved in the regulation of acid/base balance, bicarbonate rich aqueous humor secretion, the secreation of electrolytes, siliva production and bile production.
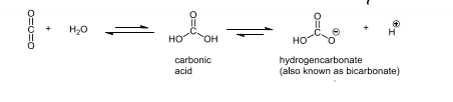
How are carbonic anhydrases involved in getting rid of CO₂?
it catalyses the reaction between water ad CO₂ to form carbonic acid, it lowers the pKa for water as it is not a good nucleophile on its own
Describe the stucture of a carbonic anhydrase
it is a metalloenzyme that contains Zn²⁺, this sits at the bottom of a 15-Å-cleft, is coordinated by 3 histidine residues, there is a water molecule or hydroxide ion boud to the 4th site. the cleft has two distict faces - one hydrophillic whch shuttles proteins in and out, and hydrophobic which binds to the substrate
Why do Mg²⁺ ions and Ca²⁺ ions have different biological functions?
Mg²⁺ ions have octahedral geometry and have a slow water exchange, Ca²⁺ ions have a irregular geometry and a fast water exchange
What is water exchange in regards to metal ions?
where the water dissociates and reassociates with the atom
How is magnesium used in DNA and RNA and sourrounding things?
2 are found in the catalytic sites of DNA polymerases, they stabilse RNA and DNA structures, critical in turning DNA on and off, tRNAs have regions known as half-crucifixs - Mg²⁺ ions are required to hold there shape, ribozymes require Mg²⁺ ions to catalyse splicing reactions
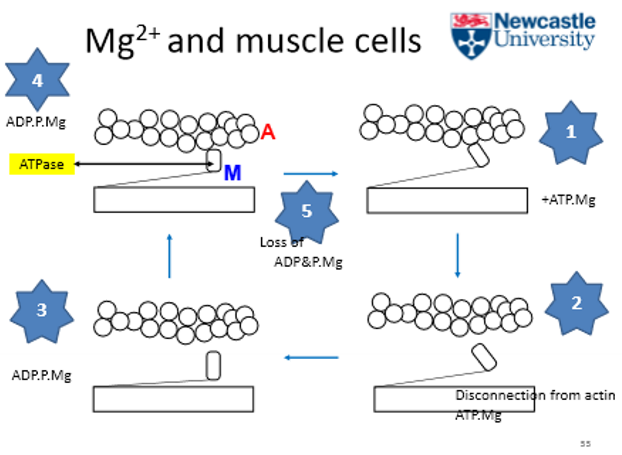
What is magnesium’s role in muscles?
they activate tention fibres
What is magnesium’s role in the cytoplasm?
anion neutralsation
How does magnesium work with phophates?
only binds to pyrophosphate and polyphosphates,
How are proteins switched on and off by kinases?
they add a phosphate group which changes the shape of the protein, phosphatases then can remove the phosphate group to change the shape back
What 3 amino acids does phosphorylation usually take place on?
serine, threonine or tyrosine
How do kinases phosphorylate amino acids?
the phosphate is supplied as the Mg-ATP complex, the Mg²⁺ ions are critical to the mechanism, ATP is bound into the protein via the adenine leaving the phosphate chain exposed or weakly bound, an anion centre on the protein helps move the trphosphate into position so the termial phosphate group can be transfered to the requisite amino acid, the protein may be phosphorylated by more than one kinase allowing convergance of cell signalling pathways
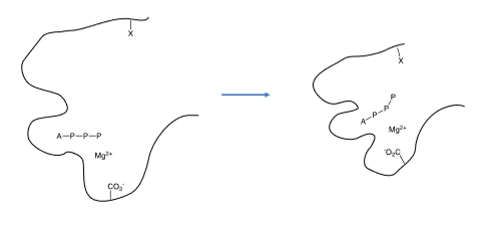
What are ribozymes?
RNA molecules that perform catalytic functions
How stable is DNA and RNA?
DNA is very stable, RNA forms a range of stable structures
What directs DNA replication?
DNA
What are the key properties of Ca²⁺ ions?
selective, it ca interact with neutral oxygen donors, bind to a number of centres at once due to its irregular coordination geometry, they readily exchange water with other ligands, the ligands on the ion are fluctional
What is calmodulin?
a small, acidic, dumbell shaped, protein involved in the mediation of Ca²⁺ signal in lots of pathways, found in some enzyme complexes, binds 4 Ca²⁺ ions through carbonyl and carboxylate groups - the binding is complex using specific lewis bases so it doesn’t stablise cross-links within the protein