organization of the basal ganglia
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
the basal ganglia are large structures that are easily identified in structural MRI scans
the basal ganglia have three input nuclei that receive projections from all parts of cerebral cortex
Among these three input nuclei, the caudate processes cognitive information, the putamen processes sensorimotor information, and the nucleus accumbens processes limbic information.
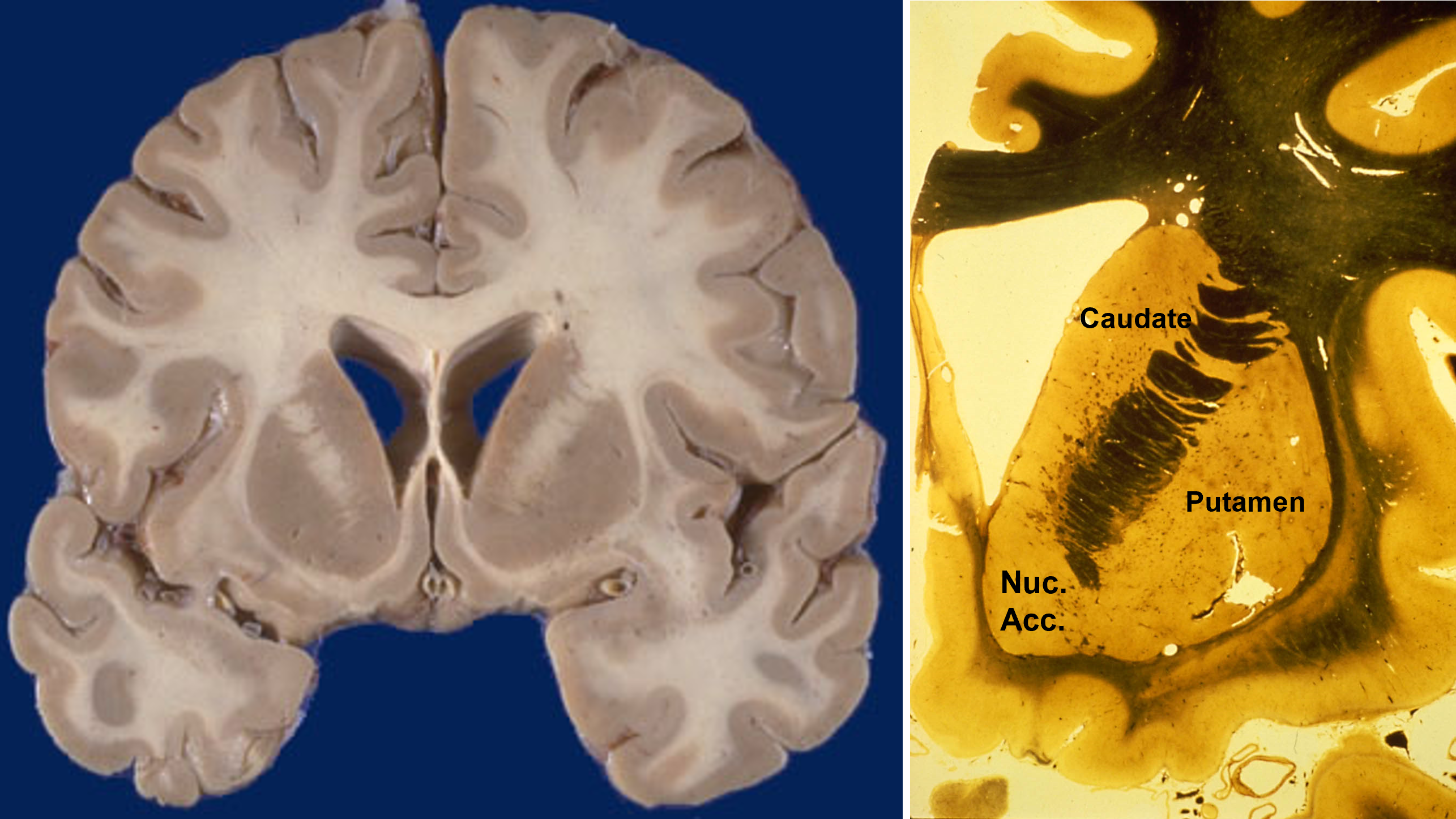
the caudate and putamen are cytologically identical and have similar embryological origins
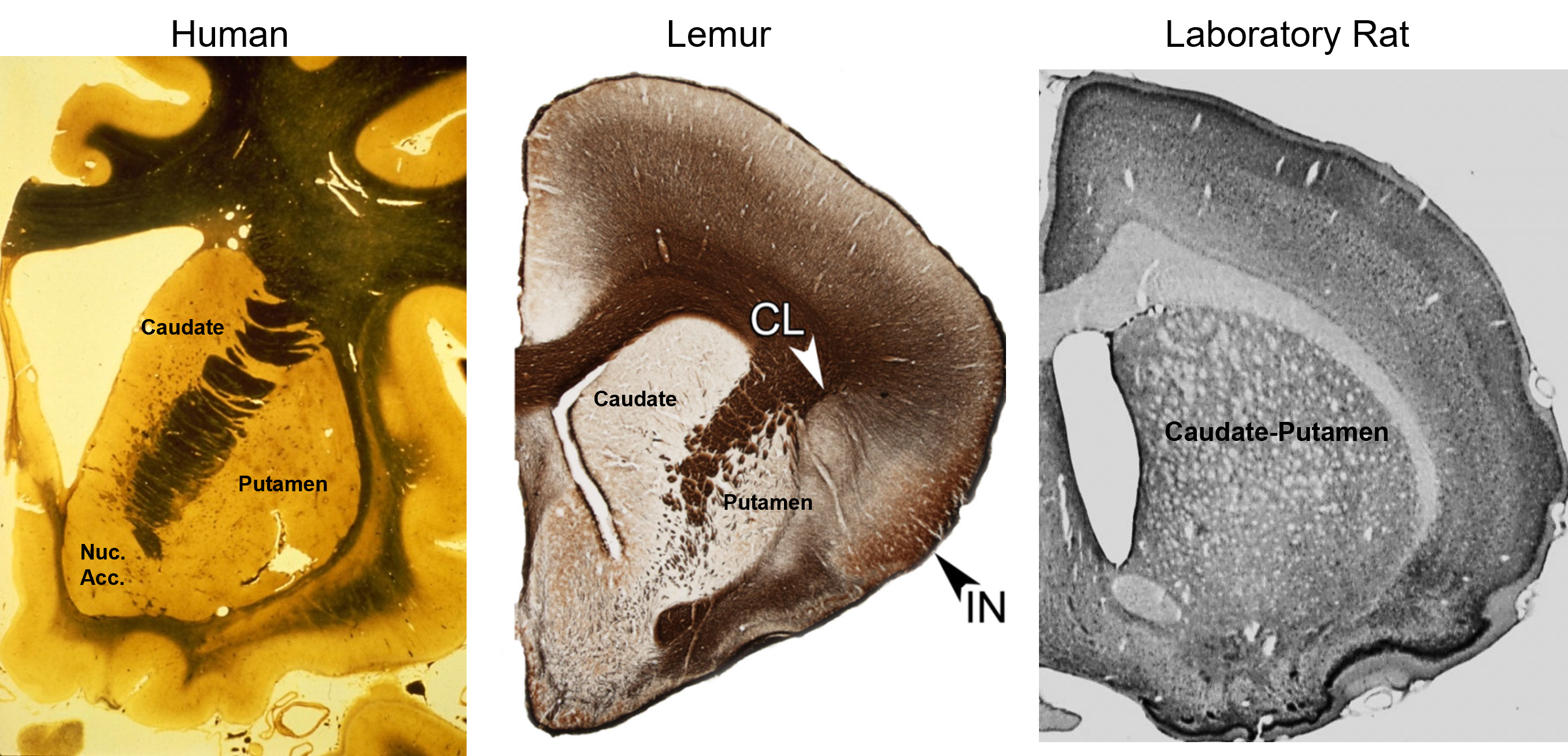
The caudate, putamen, and nucleus accumbens have identical cell types and perform similar computations, but receive inputs from different cortical regions. In primates, the separation between caudate and putamen is due to fibers growing between the cortex and thalamus during embryological development. These nuclei are not separated in rats.
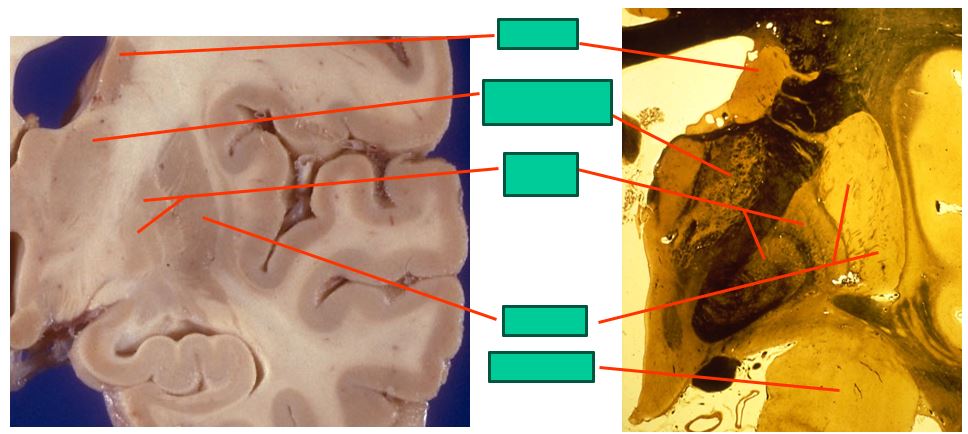
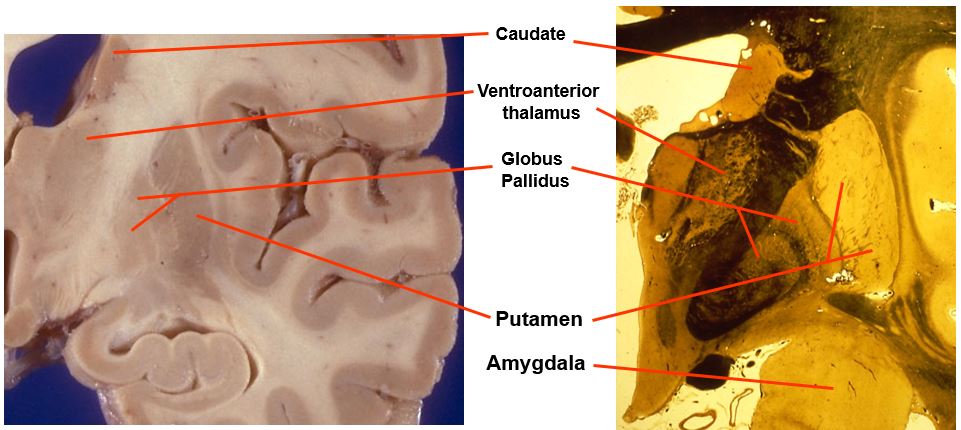
the mid-level basal ganglia looks like this
includes:
caudate
ventroanterior thalamus
globus pallidus
putamen
amygdala
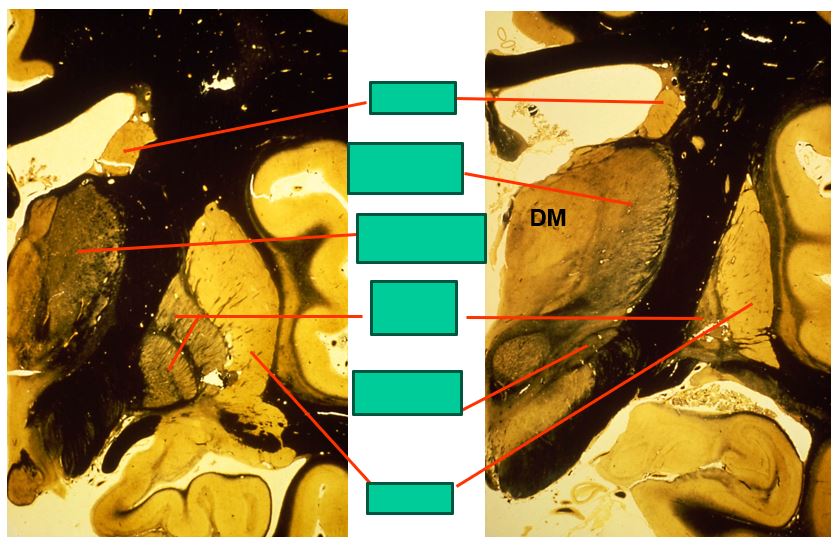
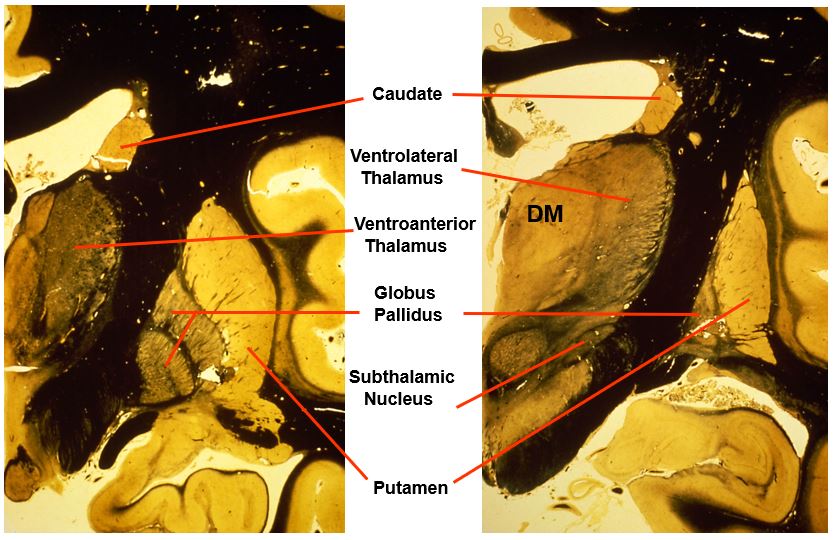
the caudal level of the basal ganglia look like this
includes:
caudate
ventrolateral thalamus
ventroanterior thalamus
globus pallidus
putamen
subthalamic nucleus
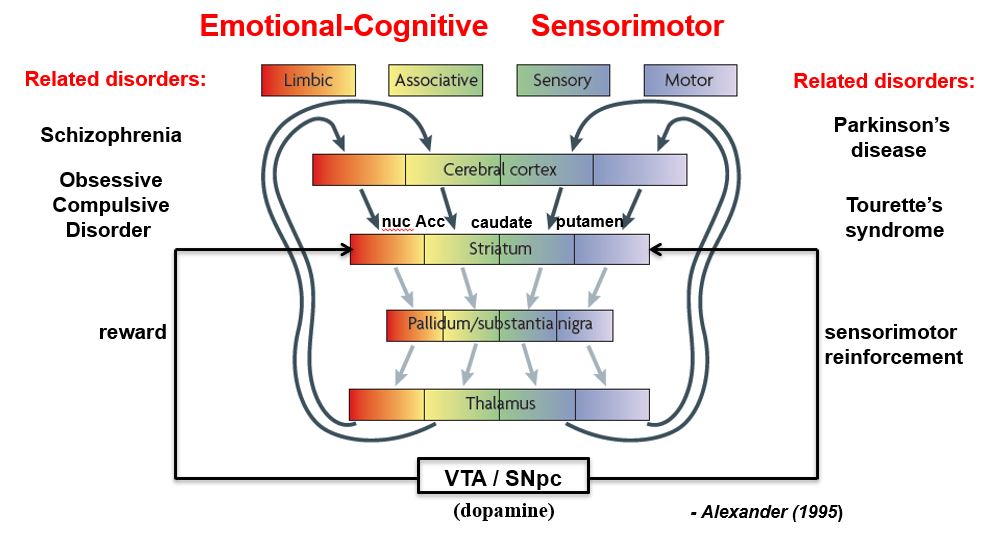
basal ganglia loops are topographically segregates to process different types of information
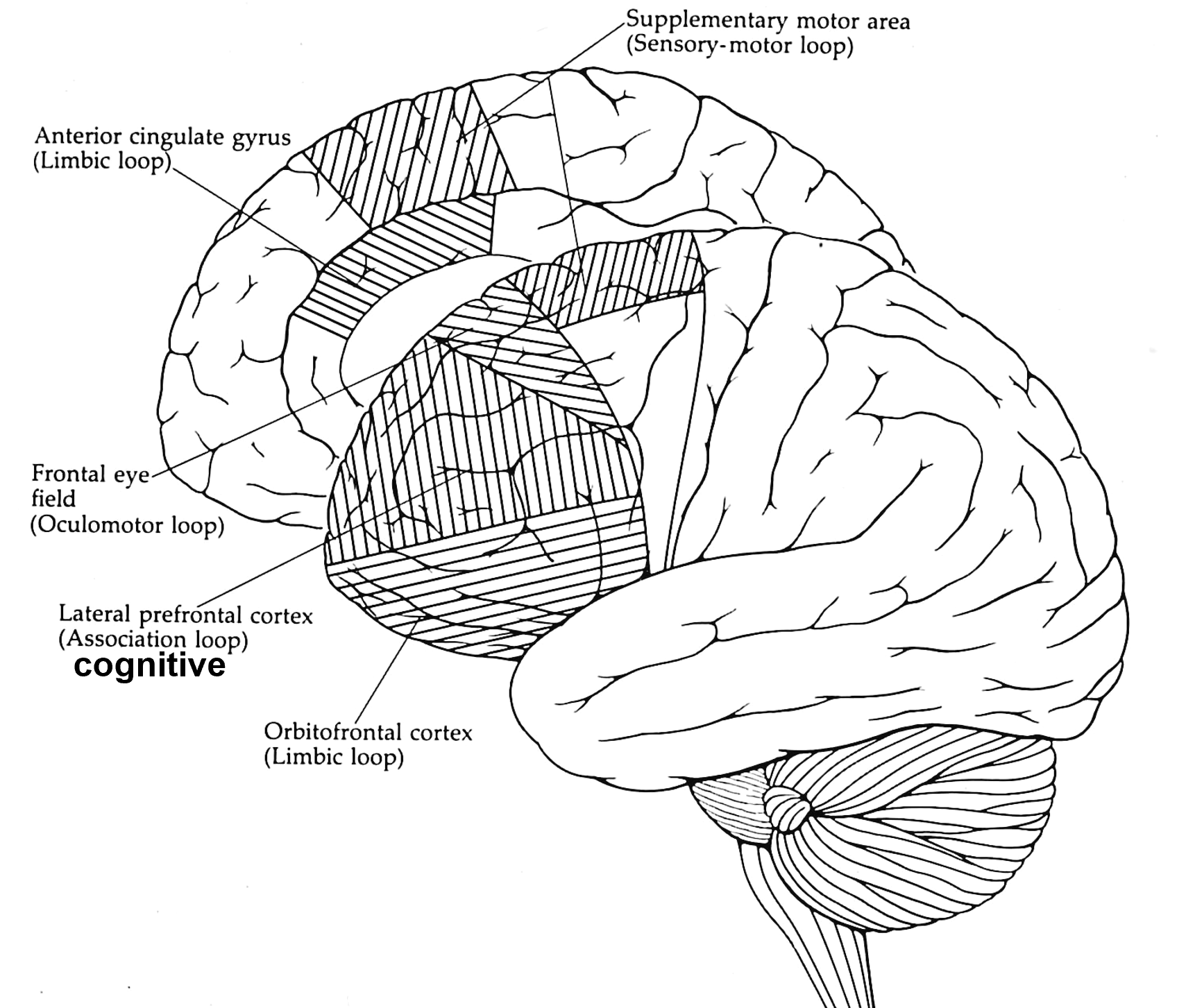
the basal ganglia-thalamocortical (BG-TC) loops are comprised of several different functional channels
Parallel channels or loops
Processes related cortical information
Corticostriatal convergence from related cortical areas
Terminate in specific frontal lobe areas
Four loops identified
sensorimotor (SI, SII, motor areas)
oculomotor (frontal eye fields)
orbitalfrontal (limbic / emotions / drives)
prefrontal (cognitive thoughts)
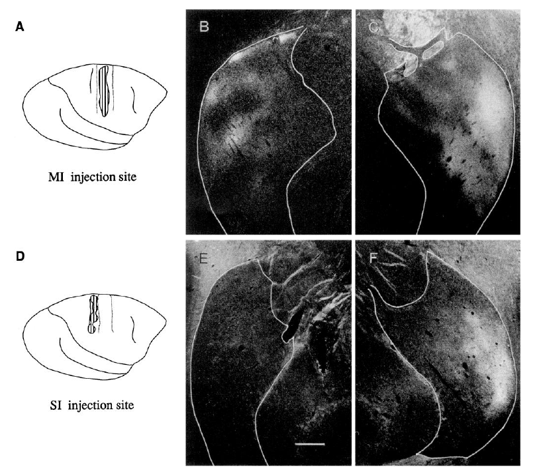
in monkeys, the putamen receives convergent inputs from the primary motor (MI) and somatosensory (SI) cortices
35S-methionine autoradiography (anterograde tracing)
Injections of anterograde tracers into MI or SI cortex revealed bilateral labeling in overlapping parts of the dorsal lateral putamen
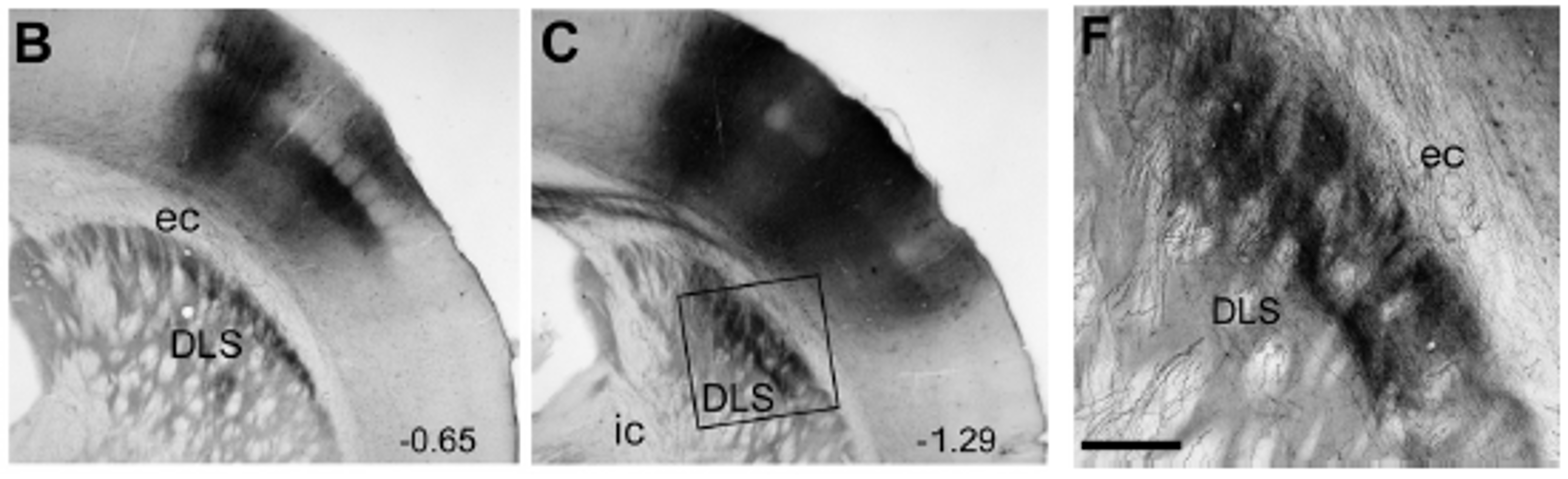
rat SI barrel cortex projects to the dorsolateral striatum (DLS)
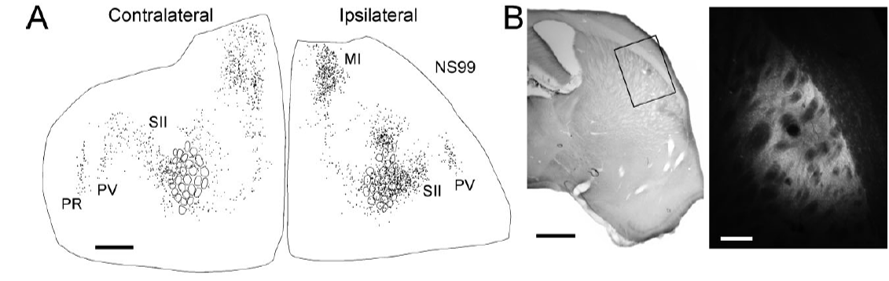
in rats, the dorsolateral receives convergent inputs from the primary motor (MI) and somatosensory (SI) cortices
the putamen (in humans) or dorsolateral striatum (in rats) is needed for the expression of sensorimotor habits
Defining characteristics of sensorimotor habits
well learned, highly repetitive behaviors
stereotyped sequences of motor activity
behaviors frequently evoked in familiar contexts
resemble a stimulus-response (S-R) association
executed automatically (almost non-consciously)
insensitive to reward devaluation
the dorsolateral striatum (DLS) in the rat homologue of the primate putamen; it mediates repetitive sensorimotor behaviors
Grooming
DLS neurons encode stereotyped grooming sequences
grooming sequences are blocked by DLS lesions
Exploratory Whisking
DLS neurons discharge rhythmically during whisking
whisker contacts with stimuli evoke stereotyped responses
resembles S-R associations
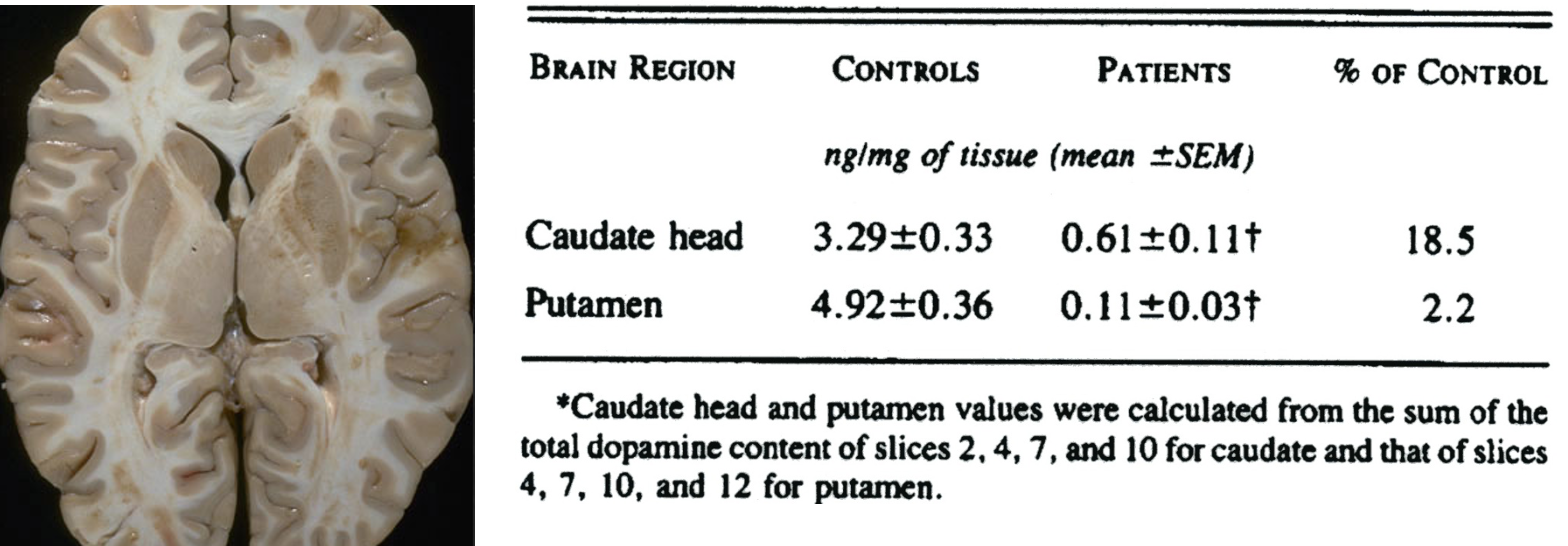
in parkinson’s disease, postmortem analysis indicates that dopamine loss is greatest in the putamen
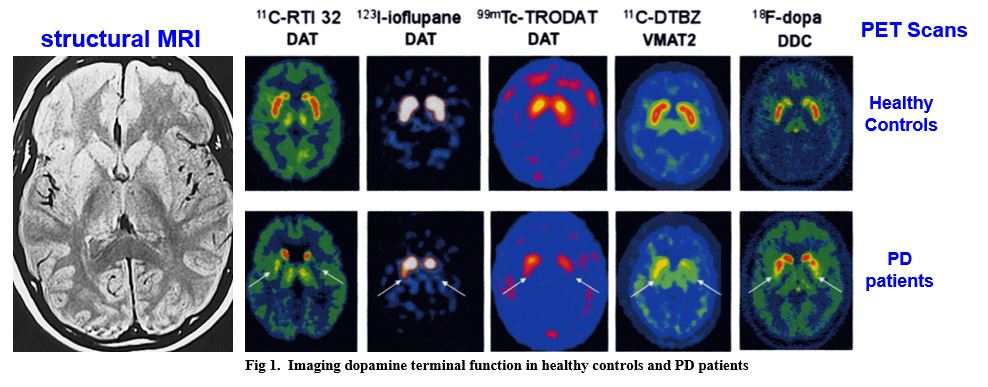
non-invasive imaging indicates that dopamine loss initially appears in the putamen of PD patients
the basal ganglia enable selection of specific behavioral and cognitive programs while suppressing competing programs
Many insights about the function of the basal ganglia have come from analysis of damage to the system and the ensuing behavioral symptoms
Ballism
uncontrollable ballistic movements
Parkinson’s Disease
poverty of movement
difficulty initiating movement
Athetosis and Chorea
involuntary movements
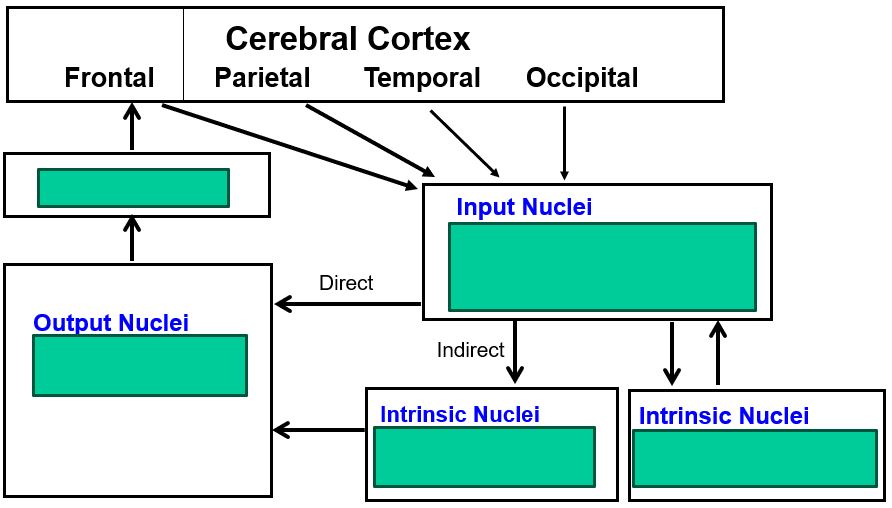
the basal ganglia contain multisynaptic pathways that proceed from cortex to the thalamus
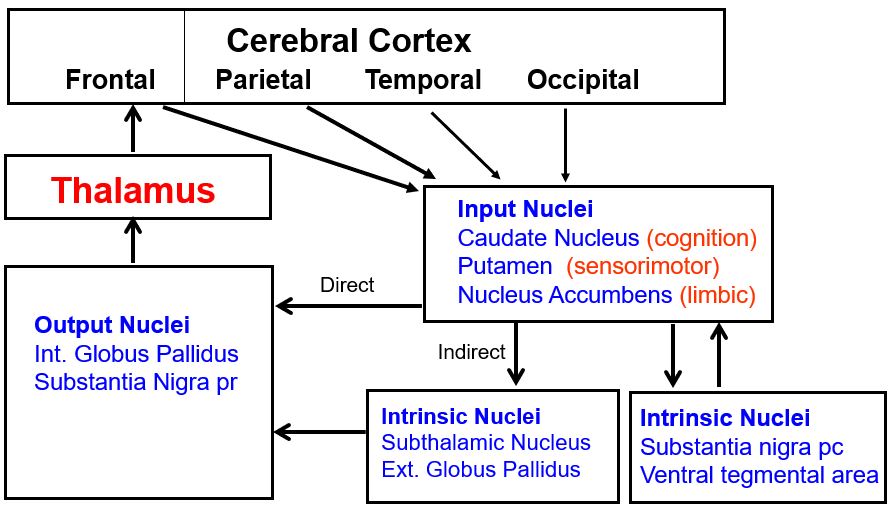
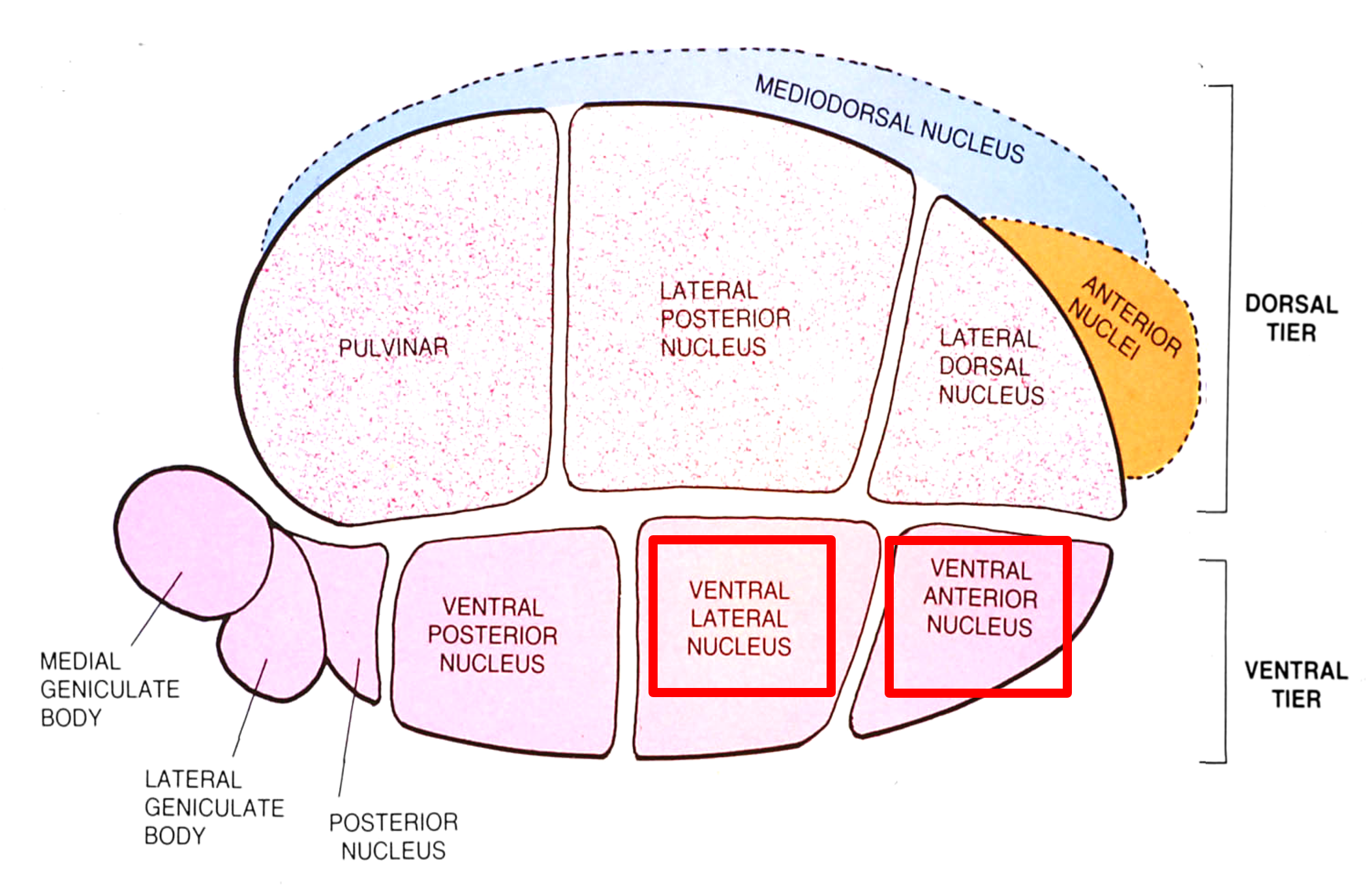
cortical processing of information from the basal ganglia and the cerebellum depends on inputs from the thalamus (part 1)
Thalamic nuclei concerned with transmitting information to motor cortex are located in the rostral part of the ventral tier, namely the ventral lateral and the ventral anterior nuclei.
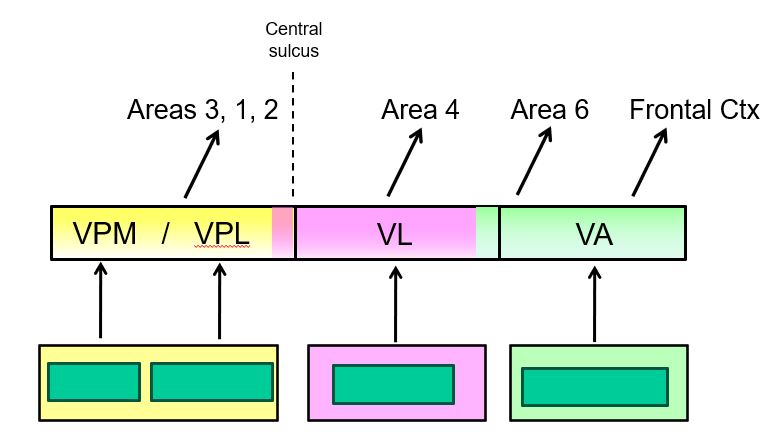
cortical processing of information from the basal ganglia and the cerebellum depends on inputs from the thalamus (part 2)
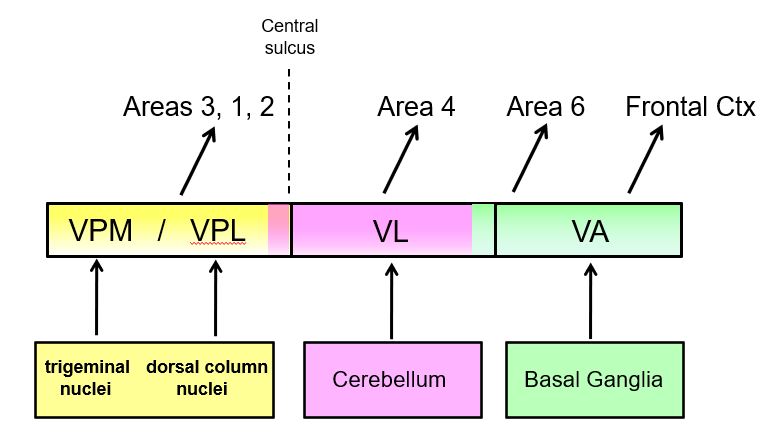
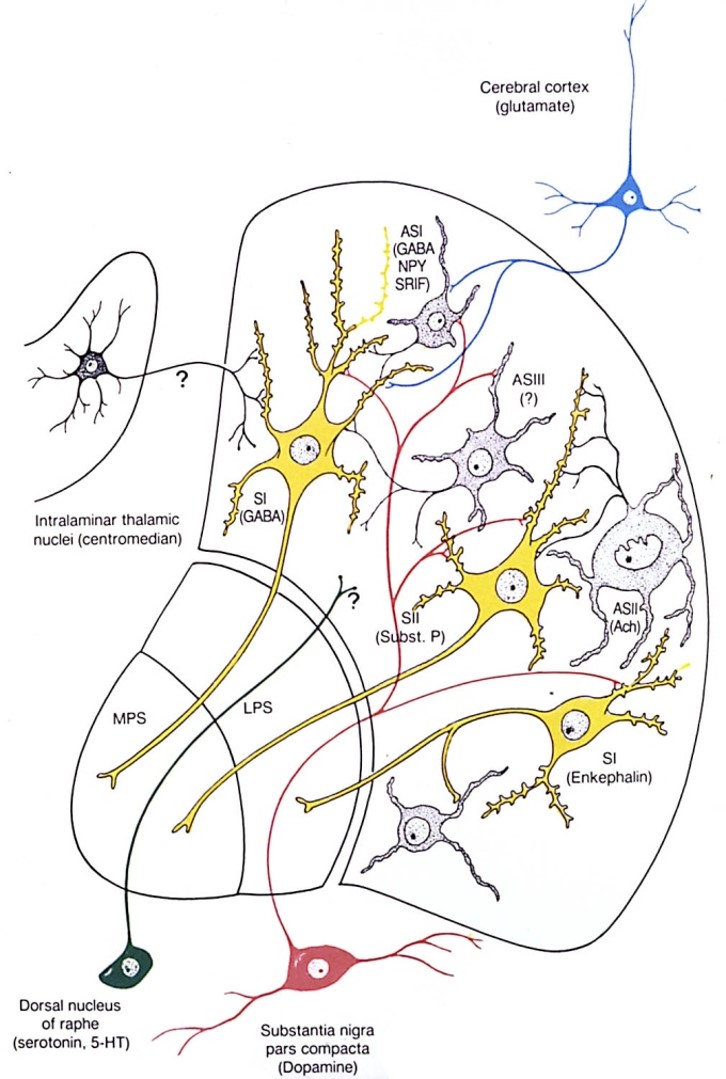
the basal ganglia receive three main types of inputs
Corticostriatal (caudate & putamen)
glutamate inputs from many cortical regions
Nigrostriatal
dopamine inputs from substantia nigra pars compacta
Thalamostriatal
parafasiculus
centromedian
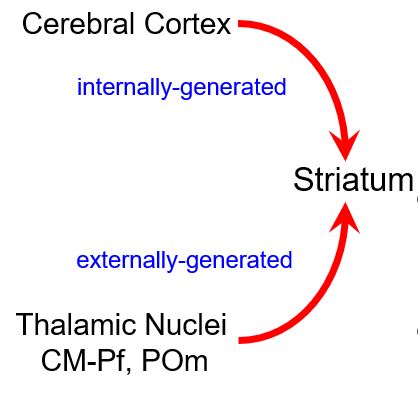
what are the differential roles of corticostriatal and thalamostriatal inputs?
Internally-generated signals
Prefrontal cortex sends the striatum intention commands
MI and SI cortex send sensorimotor context signals that accompany voluntary behavior
Externally-generated sensory signals
Sensory inputs that require re-direction of attention and possible selection of a new behavior in response to unexpected stimuli (centromedian and parafascicular nuclei)
Sensory inputs involved in the formation and maintenance of S-R associations that mediate well-learned sensorimotor habits (POm)
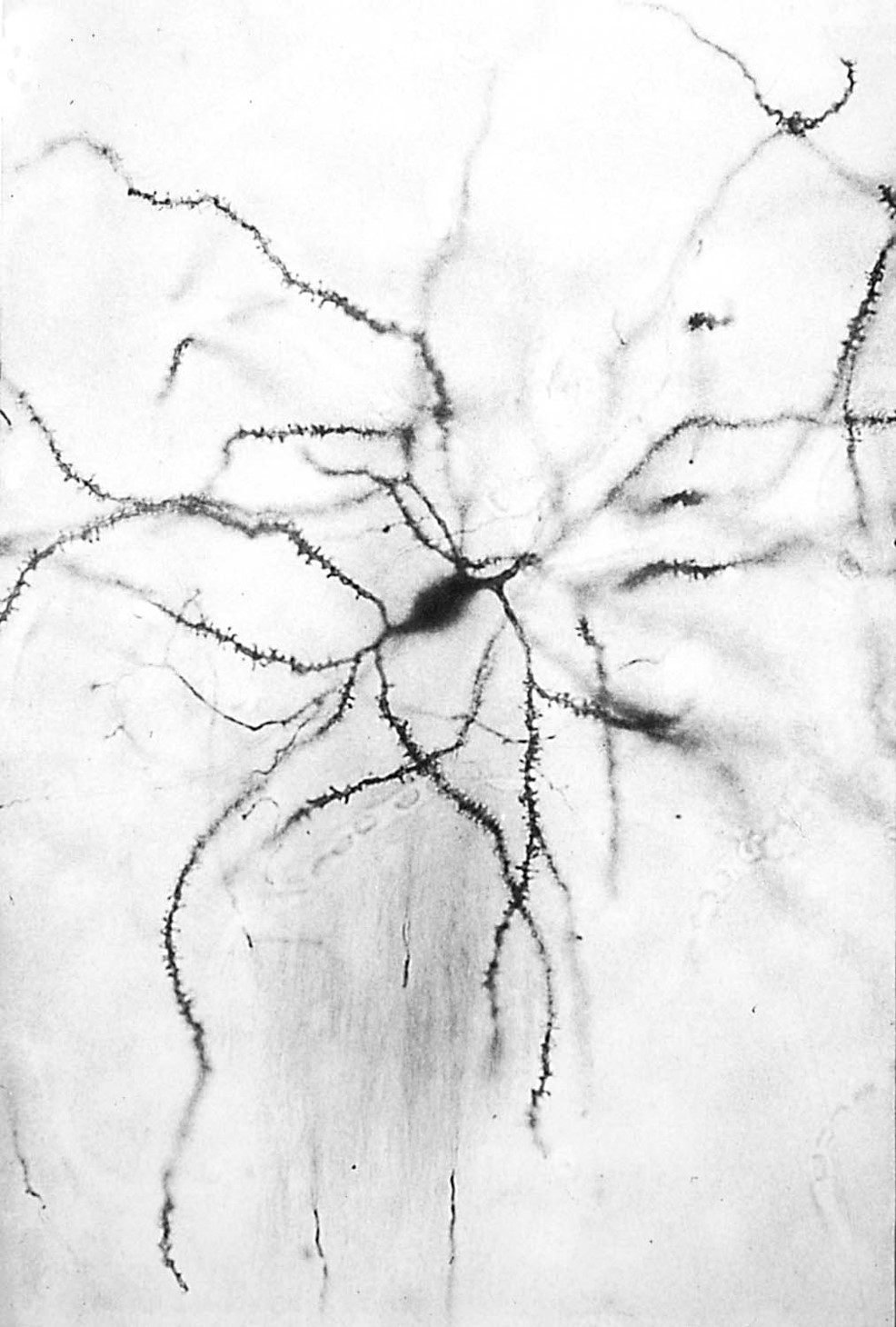
medium spiny neurons represent the main computational unit in the striatum and accumbens
Medium spiny neurons comprise > 90% of all striatal neurons and perform most of its integrative functions.
Their soma have medium diameters (~20 mm) and their dendrites are covered with spines, which are specialized for integrating synaptic inputs.
Medium spiny neurons provide the only source of output from the striatum; all other striatal neurons are local interneurons.
Medium spiny neurons use the inhibitory transmitter, GABA, co-localized with a neuropeptide.
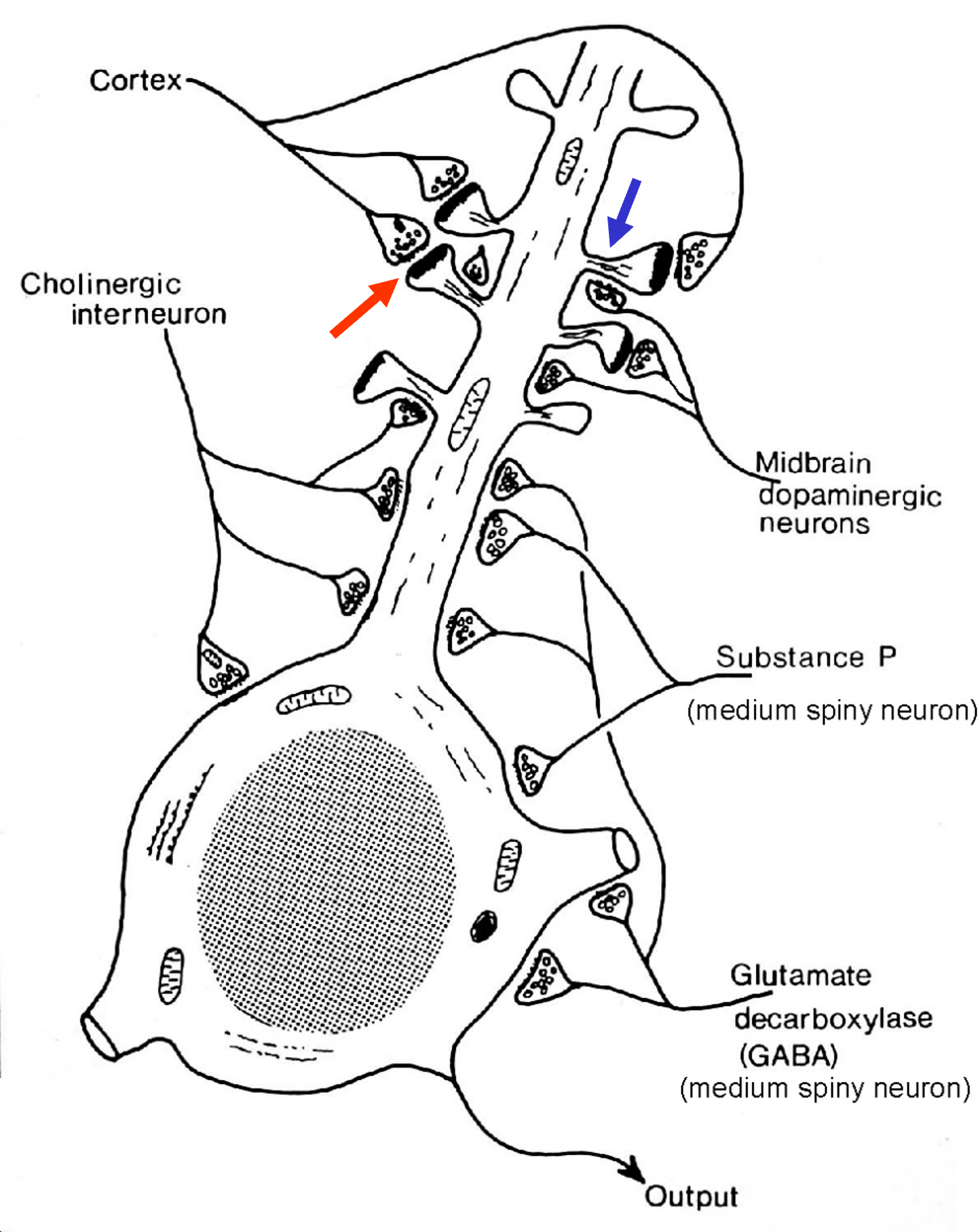
dopamine modulates the impact of corticostriatal inputs
Excitatory glutamatergic projections from the cortex terminate on the spine heads of medium spiny neurons.
Dopaminergic projections from the substantia nigra terminate on the spine necks or on more proximal parts of the dendritic shaft.
Dopamine synapses are in a location where they modulate or “gate” the effectiveness of corticostriatal excitation.
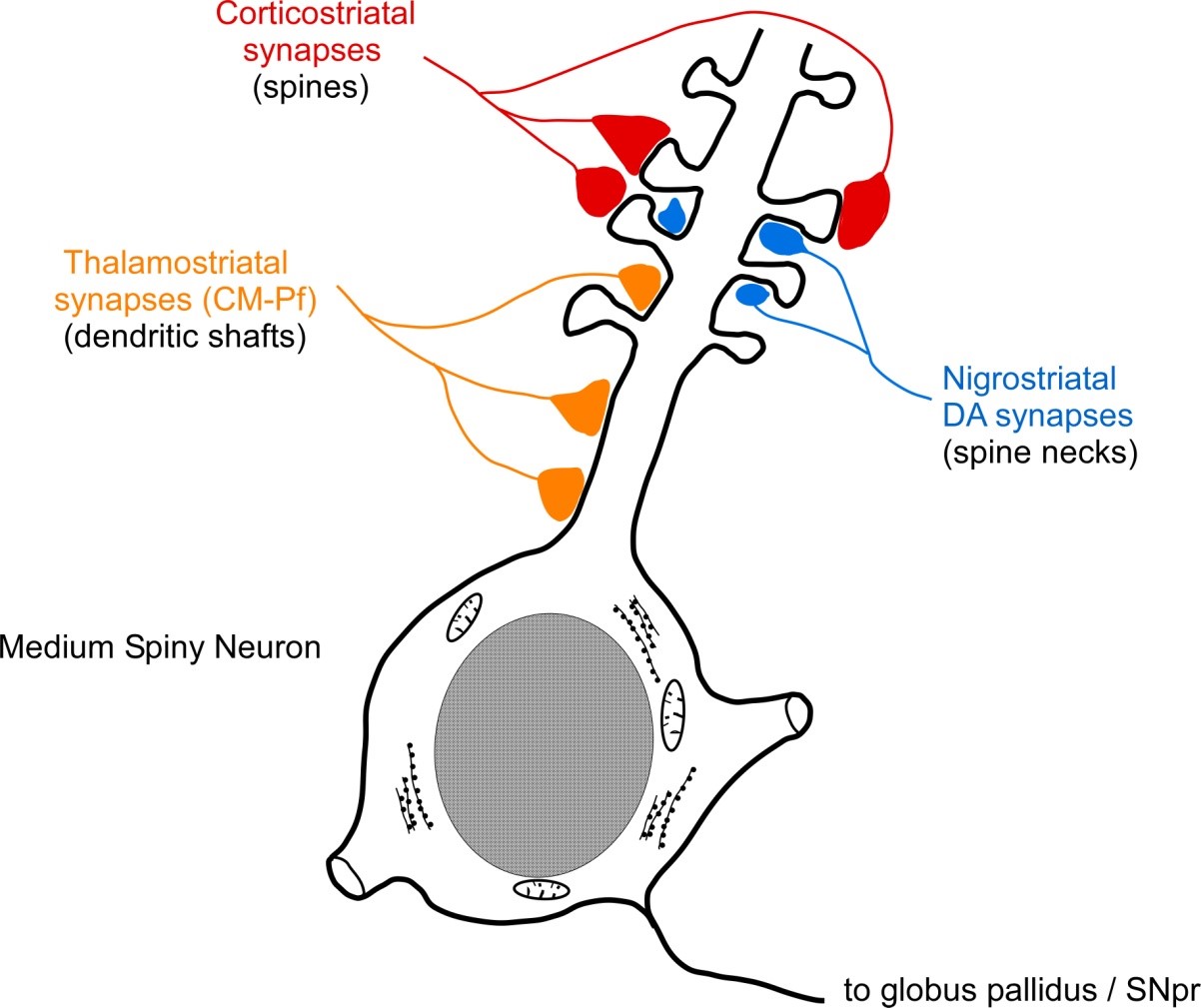
synaptic sites suggest that dopamine modulates corticostriatal interactions
Thalamostriatal synapses tend to be located on proximal dendrites. This position is less likely to be modulated by dopaminergic inputs, which are located more distally on the necks of dendritic spines.
the basal ganglia contain two parallel pathways: direct and indirect
Striatal Projections
Direct Pathway to Output Nuclei
int. globus pallidus
substantia nigra pars reticulata
GABA, substance P, dynorphin
Indirect Pathway
ext. globus pallidus
GABA, enkephalin
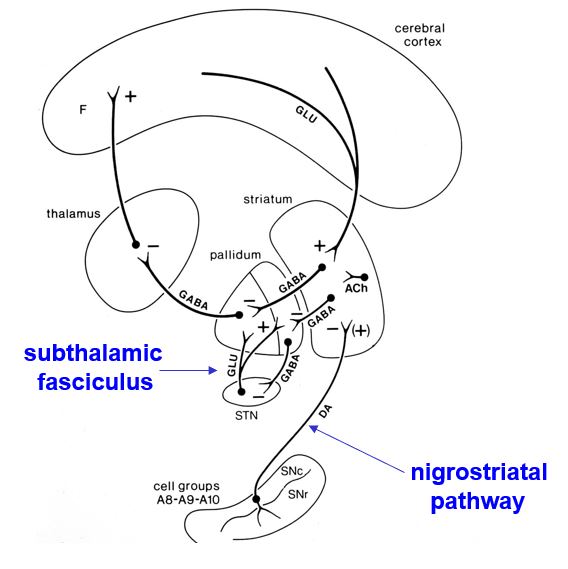
the basal ganglia contain some internuclear connections
Internuclear Connections
Subthalamic Fasciculus
GPext to subthalamic nuc.
Subthalamic nuc. to GPext
Subthalamic nuc. to GPint/SNpr
Dopaminergic Pathways
SNpc to striatum
Ventral tegmental area to Nuc. Acc.
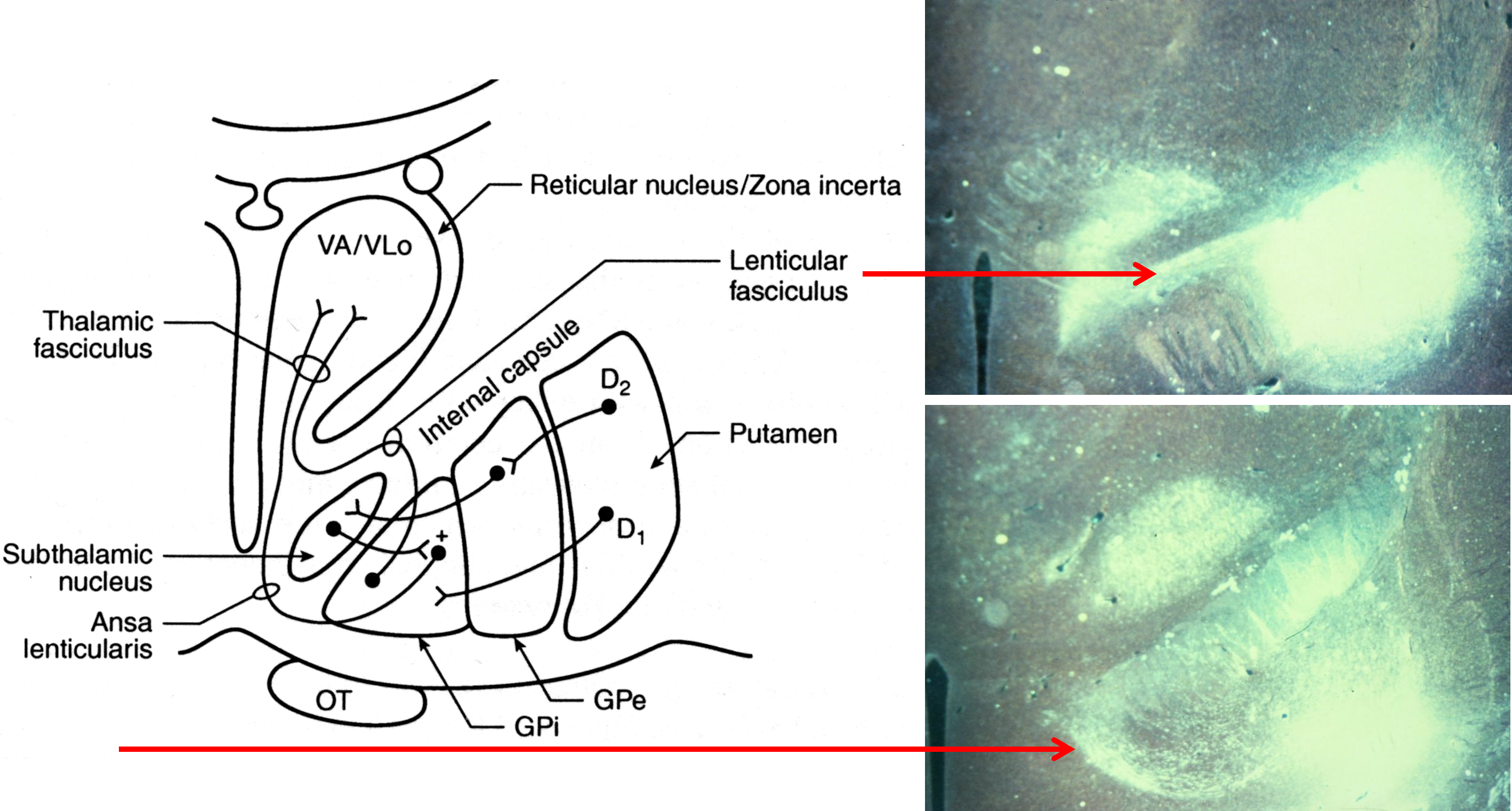
basal ganglia outputs terminate in the thalamus
Tracer injections into the medial pallidal segment indicate that this output nucleus projects to the ventroanterior and ventrolateral nuclei in the thalamus.
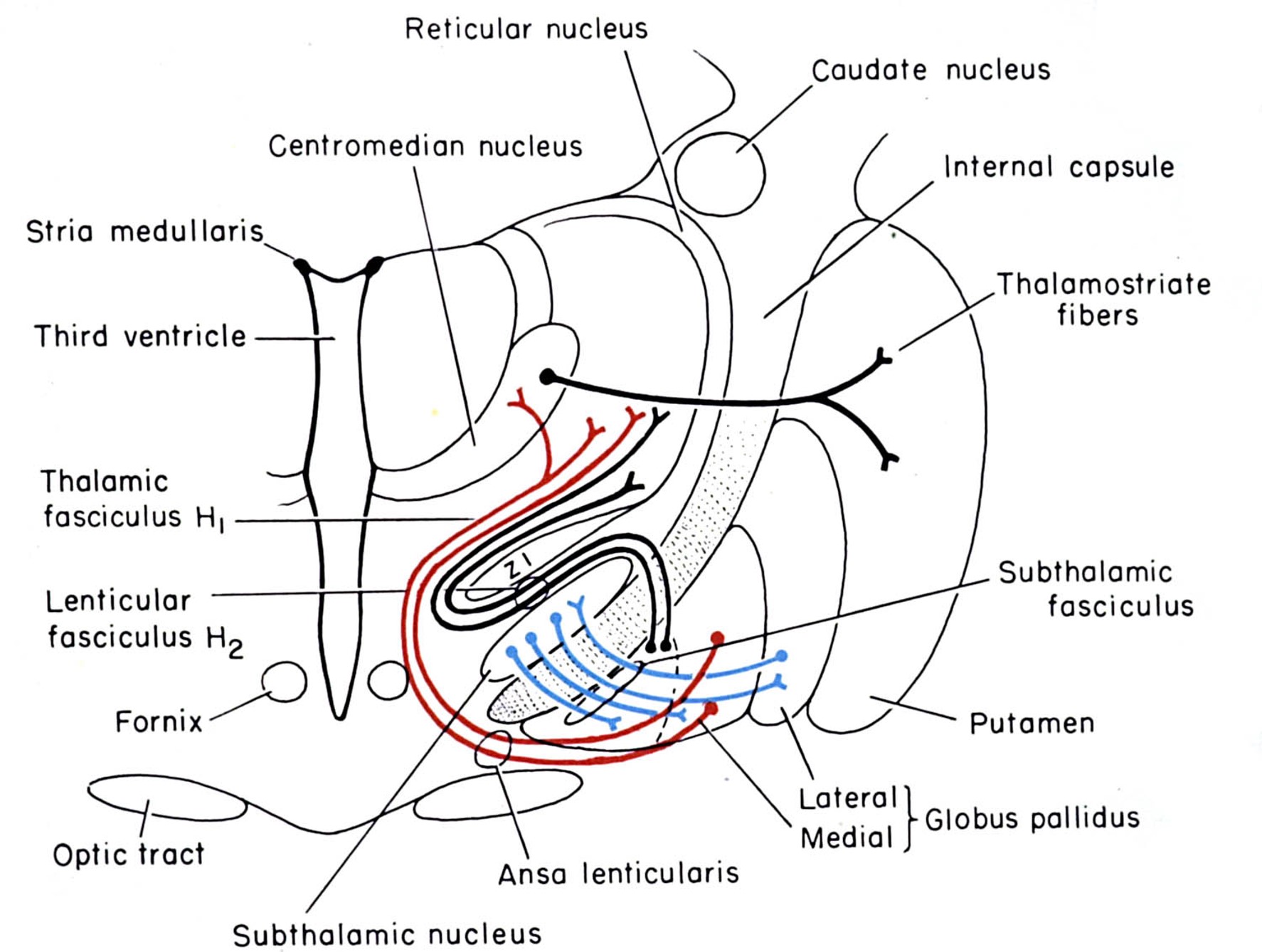
basal ganglia outputs take several routes to the thalamus
Internal Globus Pallidus
to motor thalamus (VA, VLo)
via lenticular fasciculus
via ansa lenticularis
Substantia Nigra pars reticulata
to motor thalamus (VA)
medial dorsal thalamus
pedunculopontine, sup. coll.
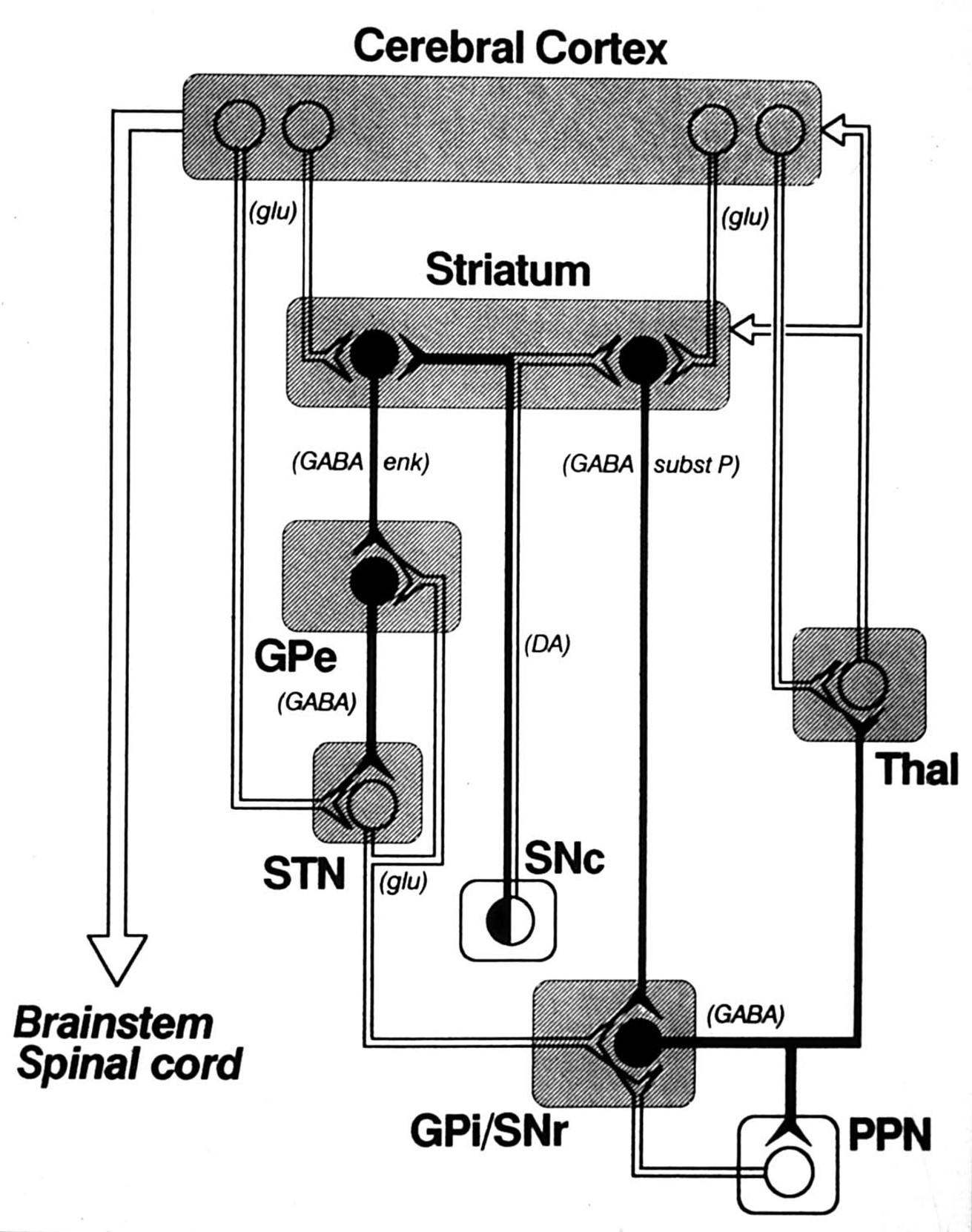
the best (but not perfect) model of the basal ganglia is the one proposed by mahlon delong
Output nuclei (GPi/SNpr) are tonically active & inhibit thalamus (VA/VL)
Opposing influences on GPi/SNpr
Direct pathway (accelerator)
Indirect Pathway (brake)
Dopamine has opposite effects on direct and indirect pathways
Disinhibition plays a key role

excessive activation of the direct pathway produces hyperkinetic disorders
Direct Pathway activation:
Increased inhibition of GPi and SNpr
Motor thalamus released from inhibition
Consistent with clinical conditions:
Hemiballism (flailing movements - contra limbs)
produced by subthalamic lesions
Huntington’s Chorea:
loss of neostriatal neurons
enkephalin colocalized with GABA
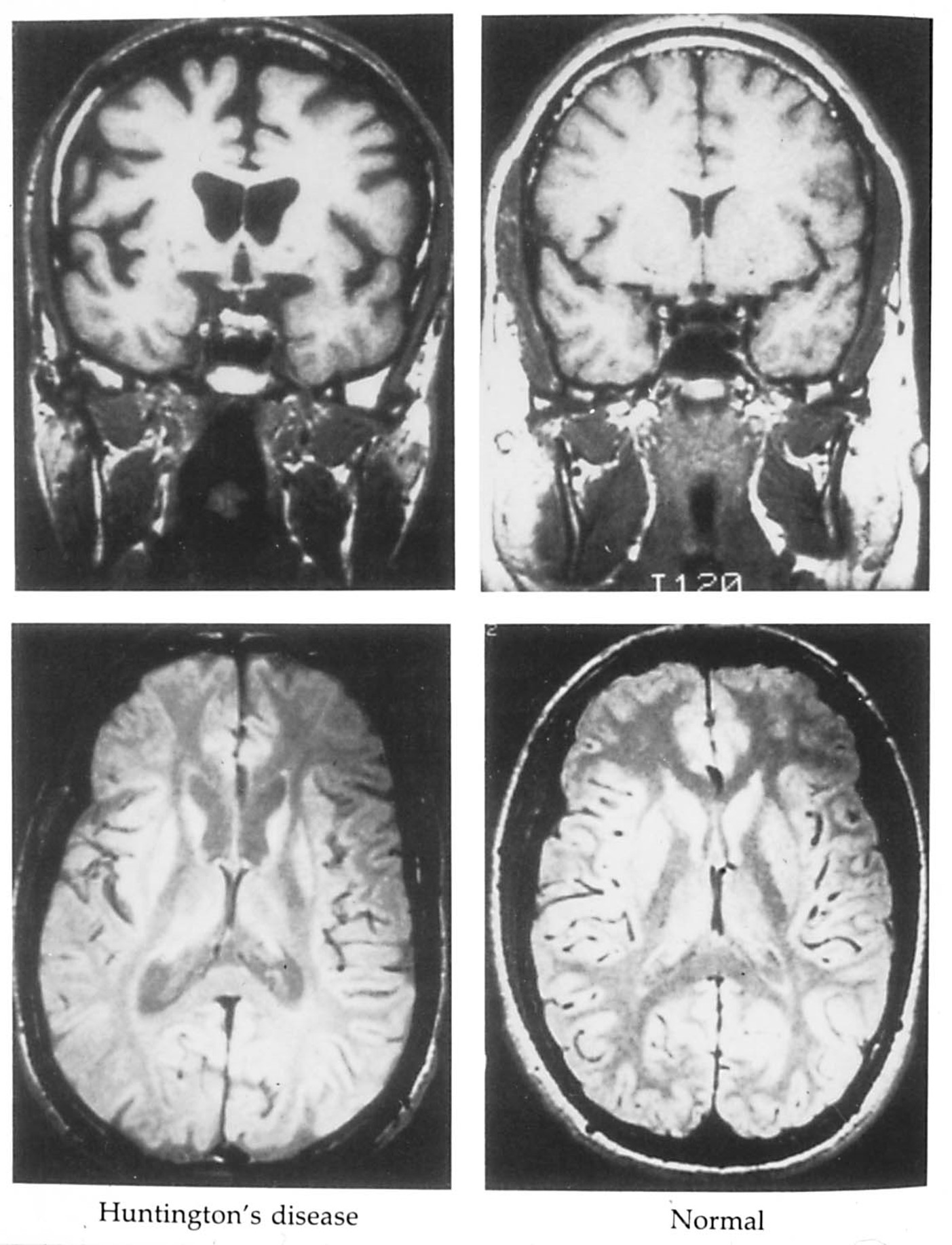
huntington’s chorea is a hyperkinetic disorder
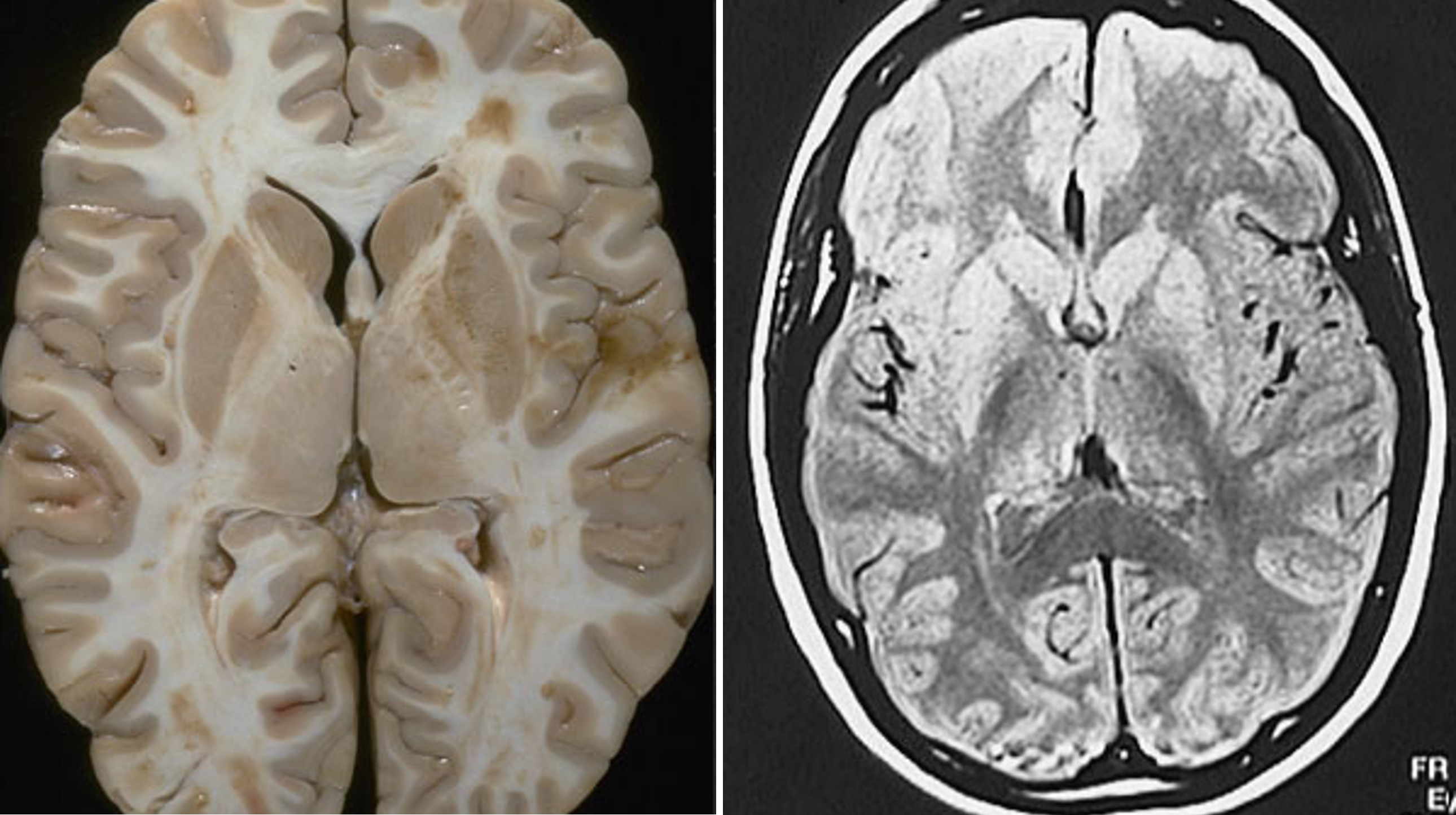
Images of the brain from a patient with Huntington’s disease reveals enlarged ventricles and shrunken caudate and putamen nuclei bilaterally.
According to the DeLong model, neuronal loss in the caudate is due primarily to the loss of neurons that use enkephalin co-localized with GABA.
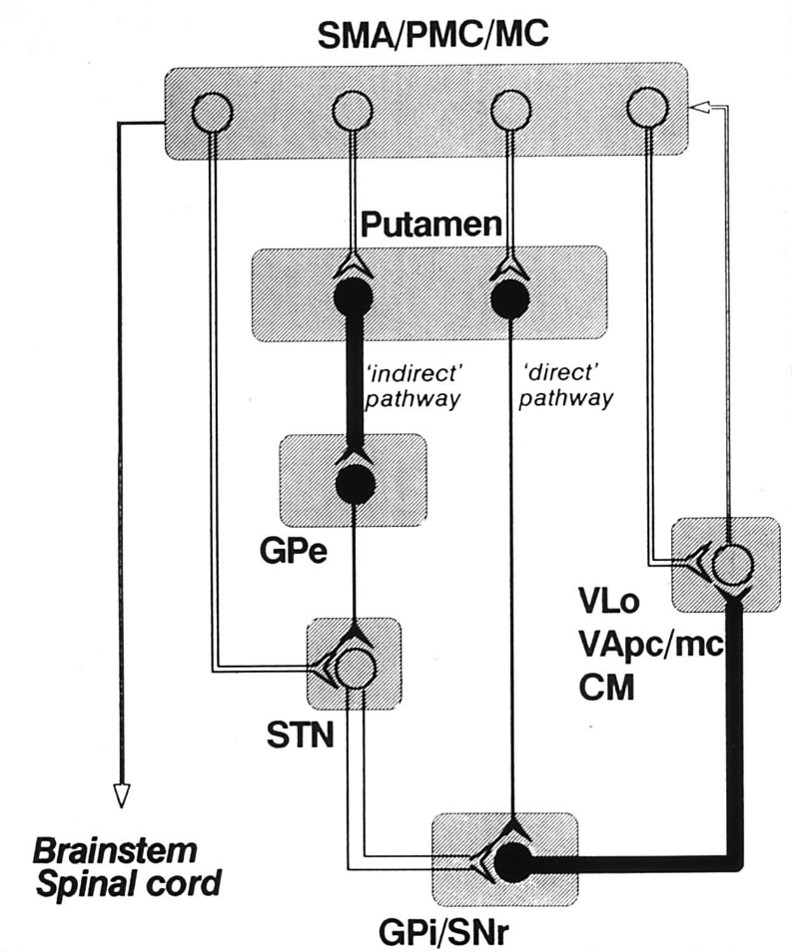
excessive activation of the indirect pathway produces hypokinetic disorders
Indirect Pathway activation
Increased inhibition of GPe
Subthalamic activity increases
Thalamus is inhibited by GPi/SNpr
Reduced thalamocortical activation of motor/premotor cortex
Consistent with clinical conditions
Parkinson’s Disease (loss of dopamine results in loss of inhibition of indirect pathway)
Experimental MPTP treatment
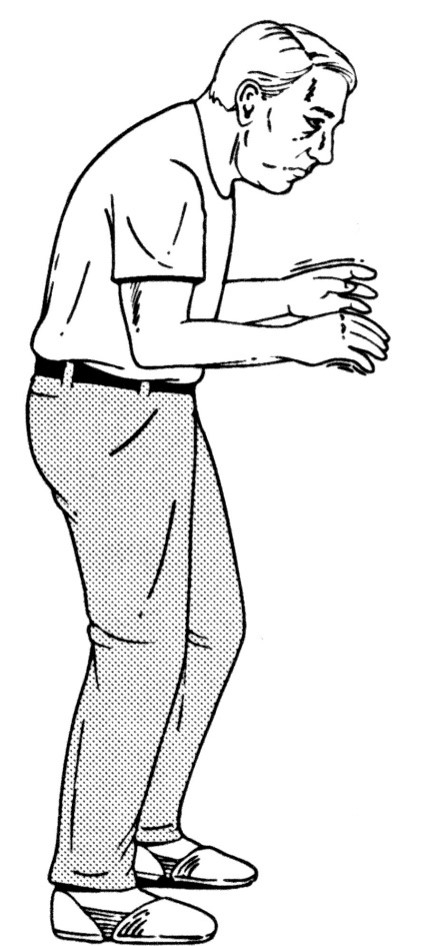
parkinson’s disease is a hypokinetic disorder
Akinesia
poverty of movement
difficulty initiating movement
Bradykinesia
slow, shuffling gait
Masked facial expression
unblinking (reptilian) stare
Paralysis agitans
tremor at rest (4-6 Hz)
Chronic, progressive degeneration
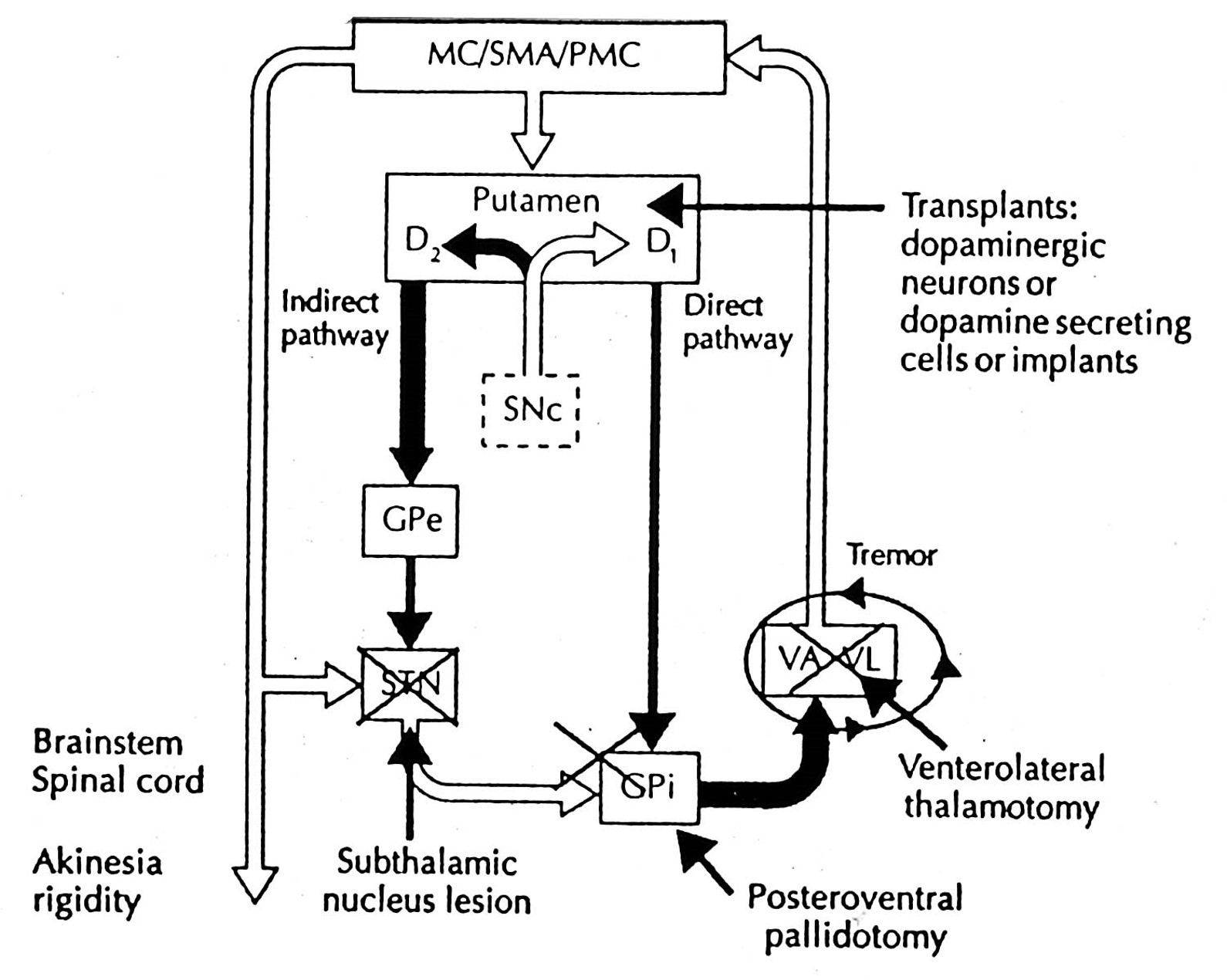
the anatomy of the basal ganglia has prompted several strategies for treating parkinson’s disease
Transplants
fetal tissue (substantia nigra)
dopamine cells in adult kidney (adrenal medulla)
Surgical Ablation
ventral globus pallidus
VA, VL thalamus (tremor)
Microelectrode Stimulation
subthalamic stimulation
what is a potential problem with pallidotomy?
A potential problem with a ventral pallidotomy is that the optimal site for the lesion is located close to the optic tract and it may produce homonymous hemianopsia.
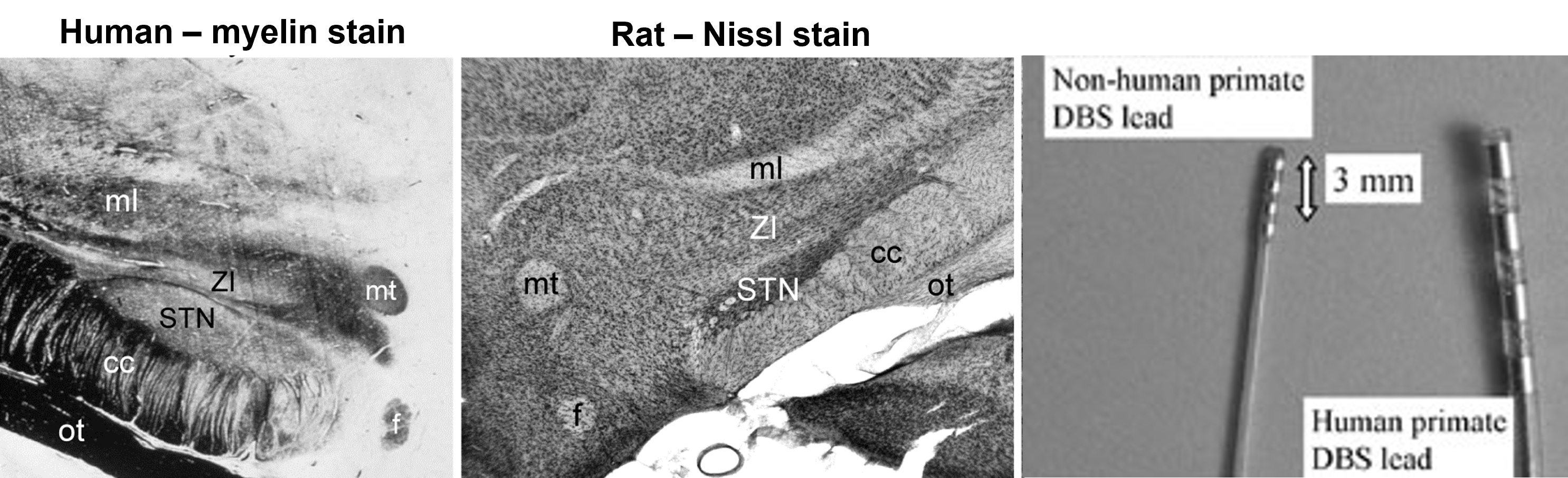
chronically implanted electrodes targeting the STN to treat Parkinson’s disease will also stimulate the zona incerta