Chapter 11 Chemistry H
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
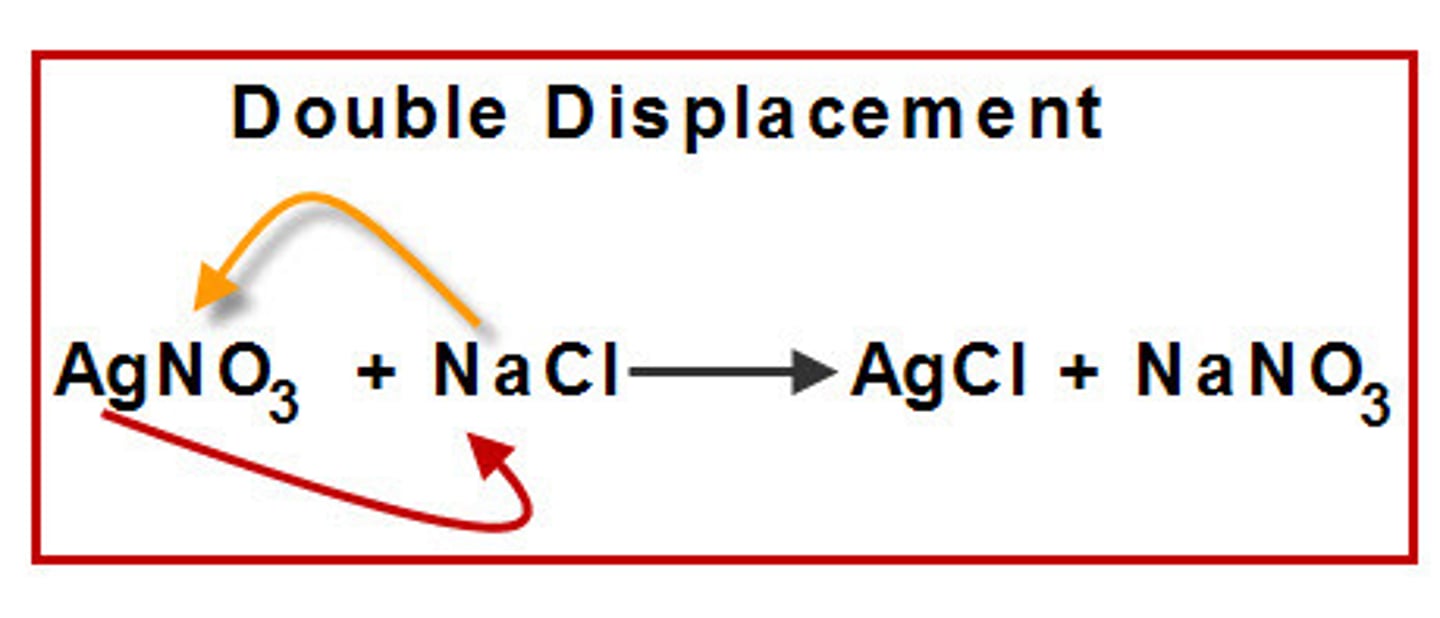
NaCl + AgNO3 --> NaNO3 + AgCl (s)
skeleton equation for aqueous sodium chloride reacting with aqueous silver nitrate to produce aqueous sodium nitrate and solid silver chloride
catalyst
a substance that speeds up a reaction without being used up in the reaction

MnO2
2H2O2 uses MnO2->2H2O + O2
what is the catalyst?

balanced equation
a chemical equation in which each side of the equation has the same number of atoms of each element
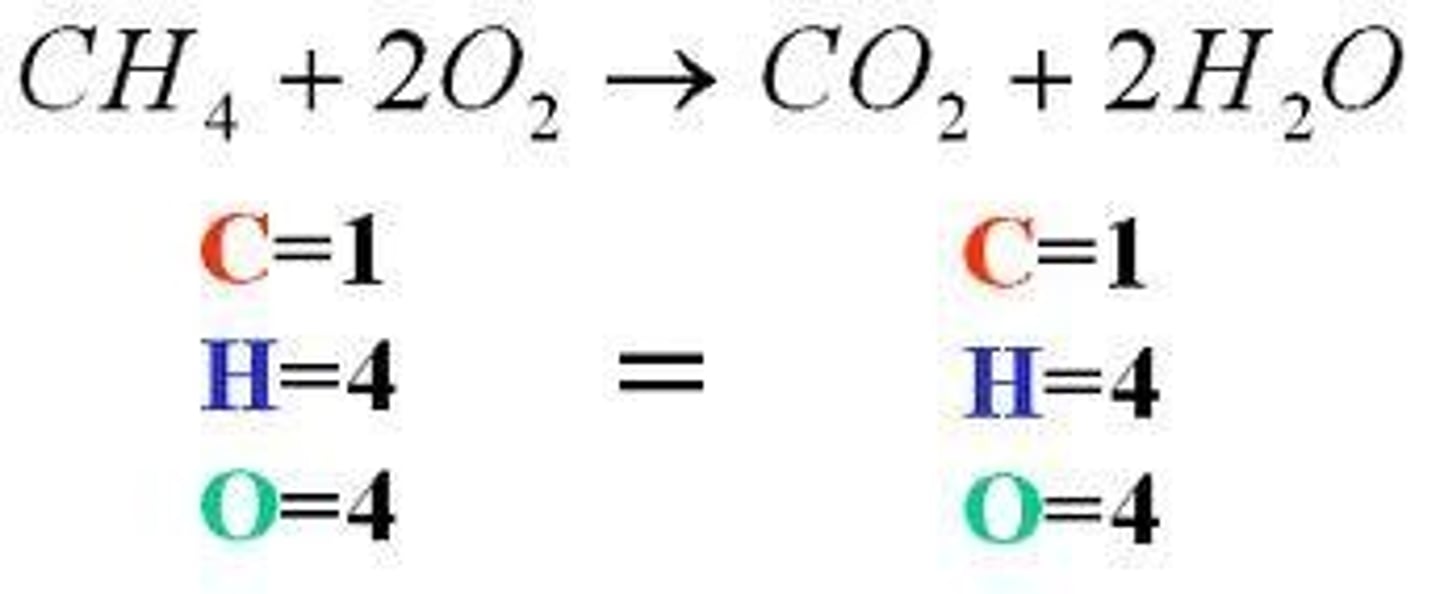
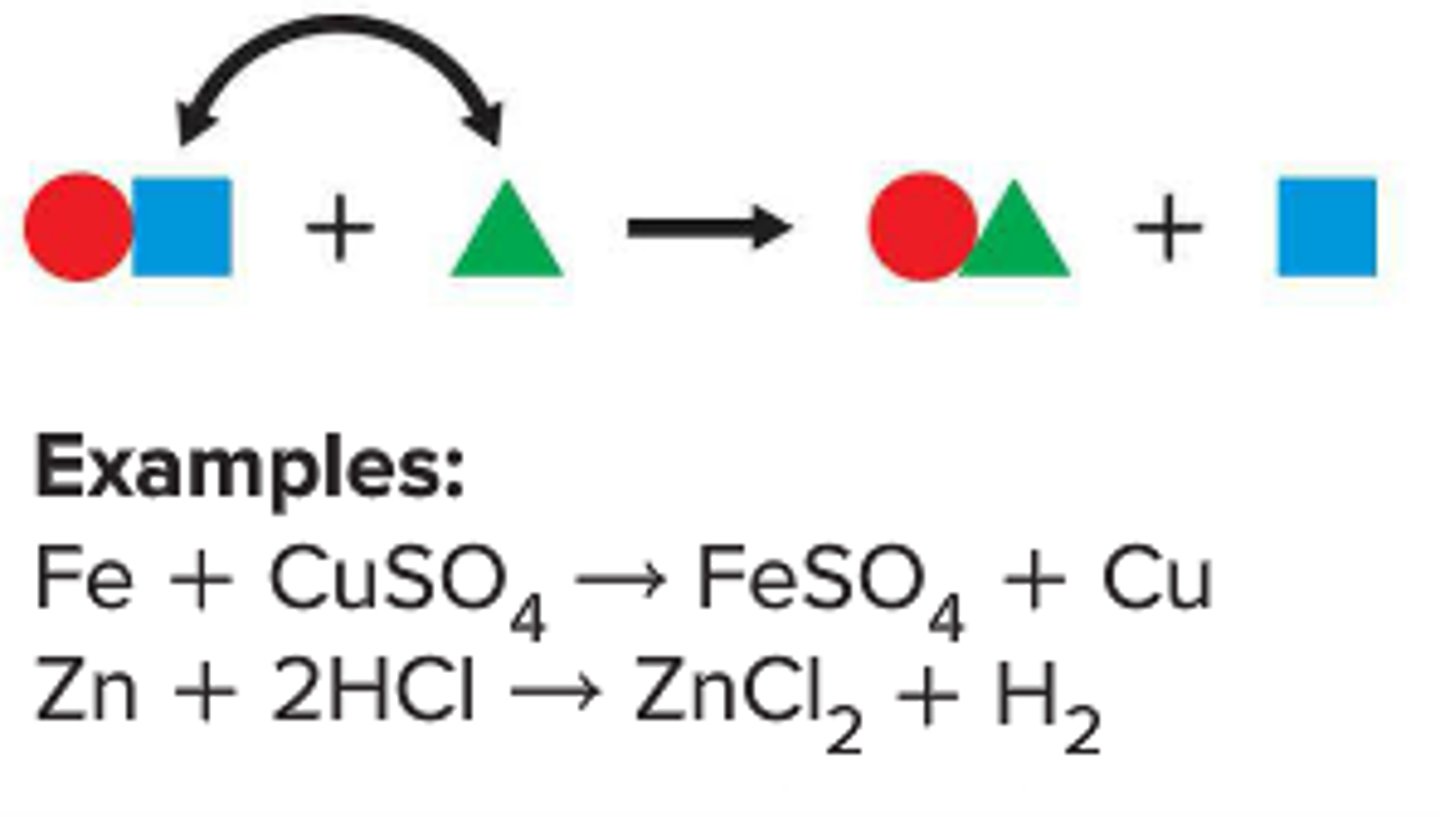
combination reaction
a chemical change in which two or more substances react to form a single new substance
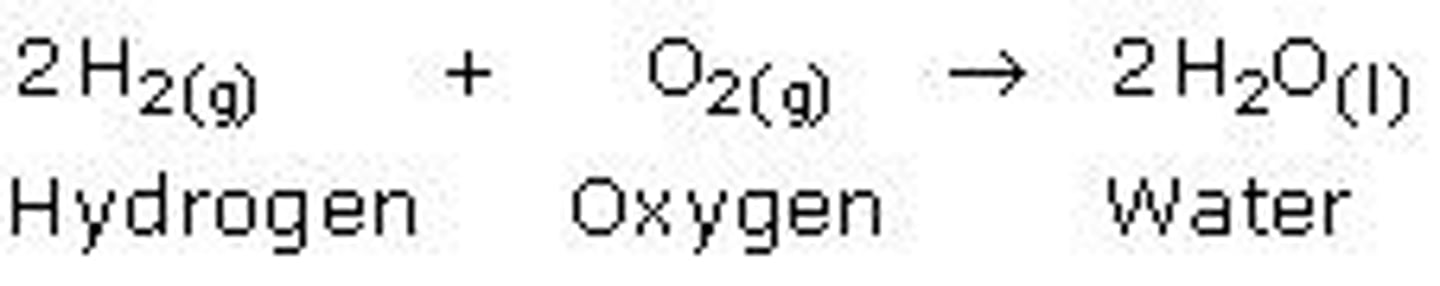
decomposition reaction
a chemical change in which a single compound is broken down into two or more simpler products
single-replacement reaction
a reaction in which one element replaces a second element in a compound
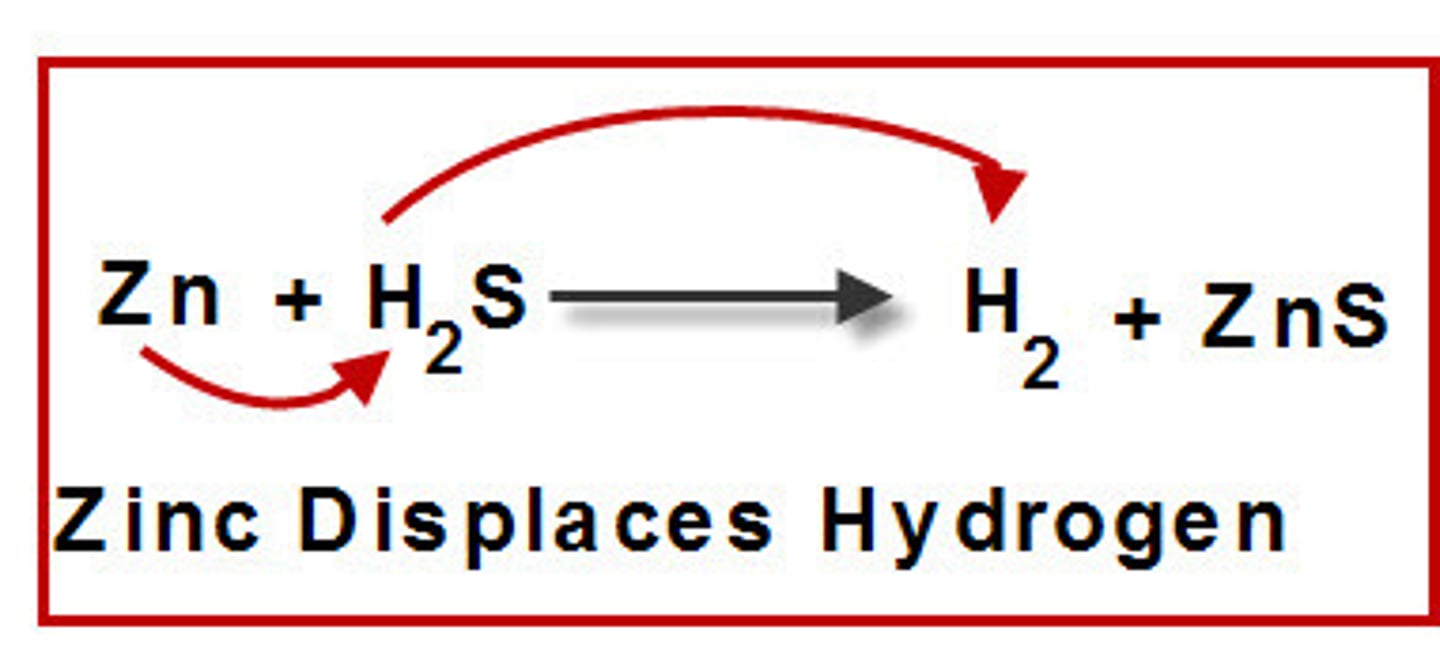
activity series
a list of metals in order of decreasing activity
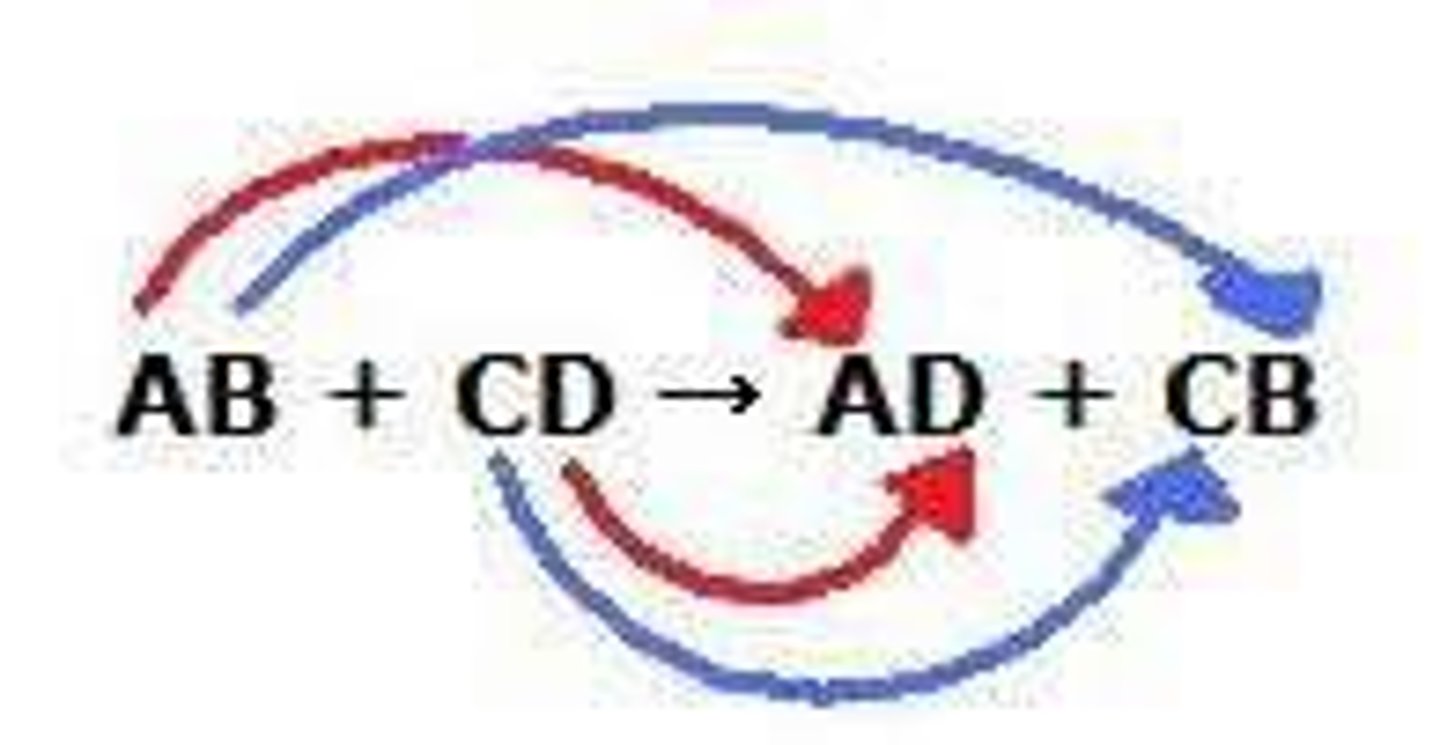
double-replacement reaction
a chemical change that involves an exchange of positive ions between two compounds
combustion reaction
a chemical change in which an element or a compound reacts with oxygen ,often producing energy in the form of heat and light

net ionic equation
an equation that shows only those particles that actually take part in the reaction

spectator ion
an ion that is not directly involved in a chemical reaction
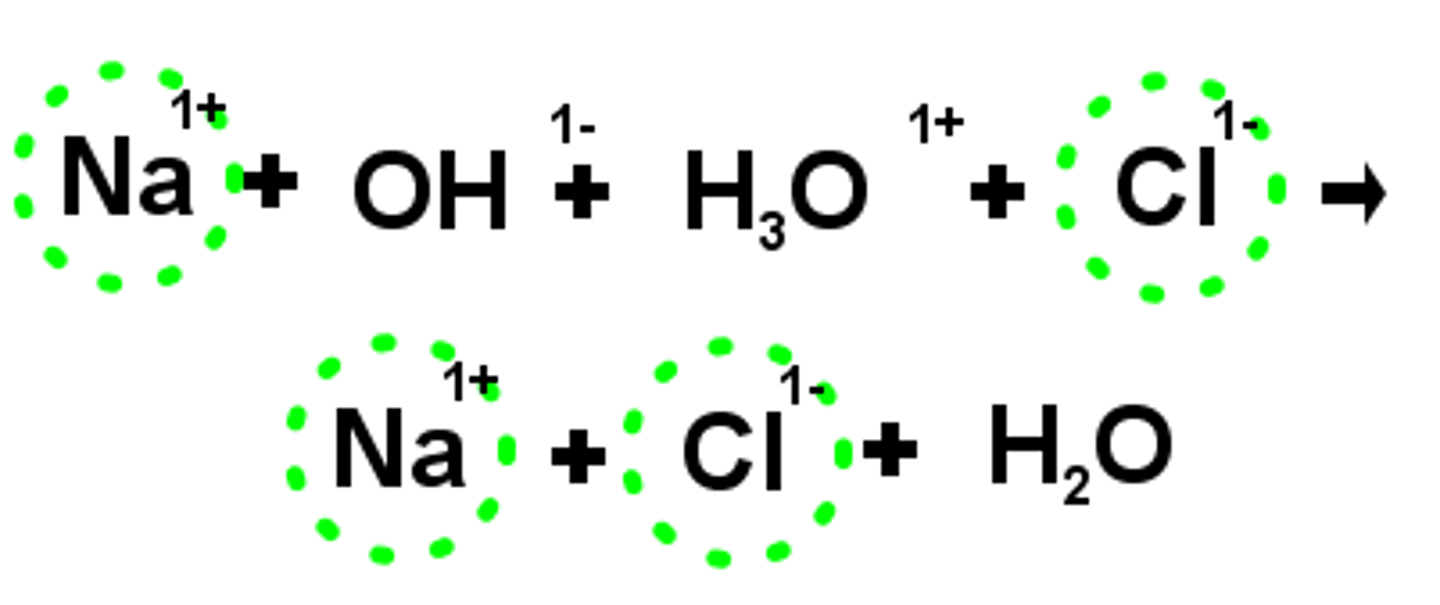
2 and single-replacement reaction
Mg +__HCl -> MgCl2 + H2
what is the coefficient for HCl that balances the reaction?
what type of reaction is this?
2 and Combination reaction
Fe + O2 -> ____Fe2O3
What is the coefficient of Fe2O3 that balances the reaction?
What type of reaction is this?

KI potassium iodide
What binary compound decomposes to form K + I2?

copper must be above silver in the activity series
Cu + 2AgNO3 -> CuNO3 + 2Ag
what condition must be true to make this reaction possible?

water
product of combustion reactions

Ca - calcium
could replace copper, iron, or silver as an ion from a compound in aqueous solution
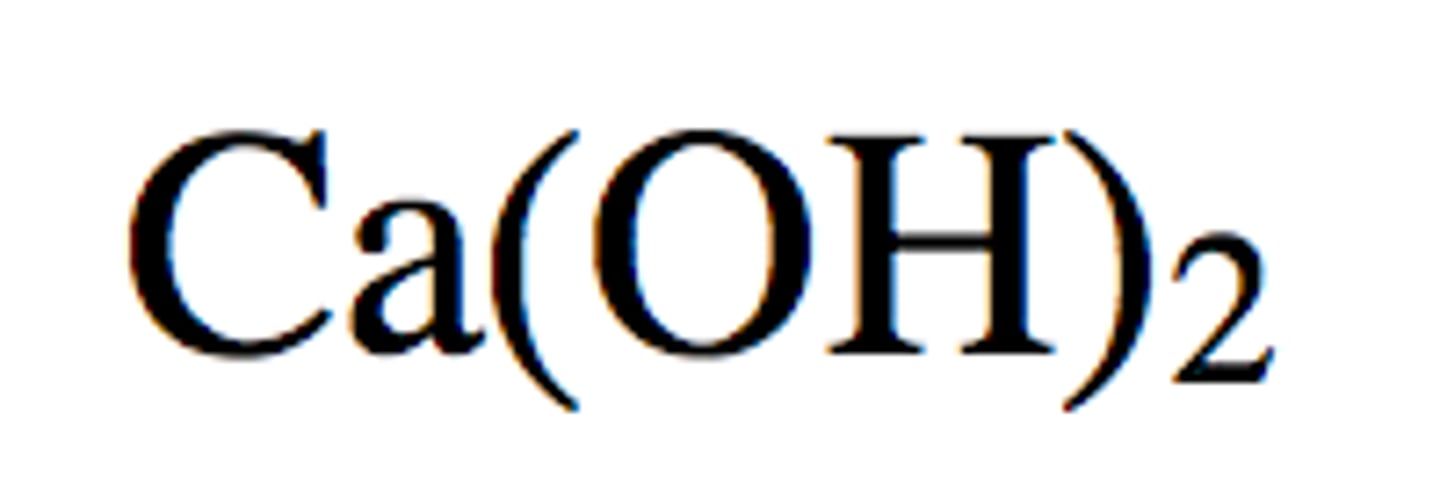
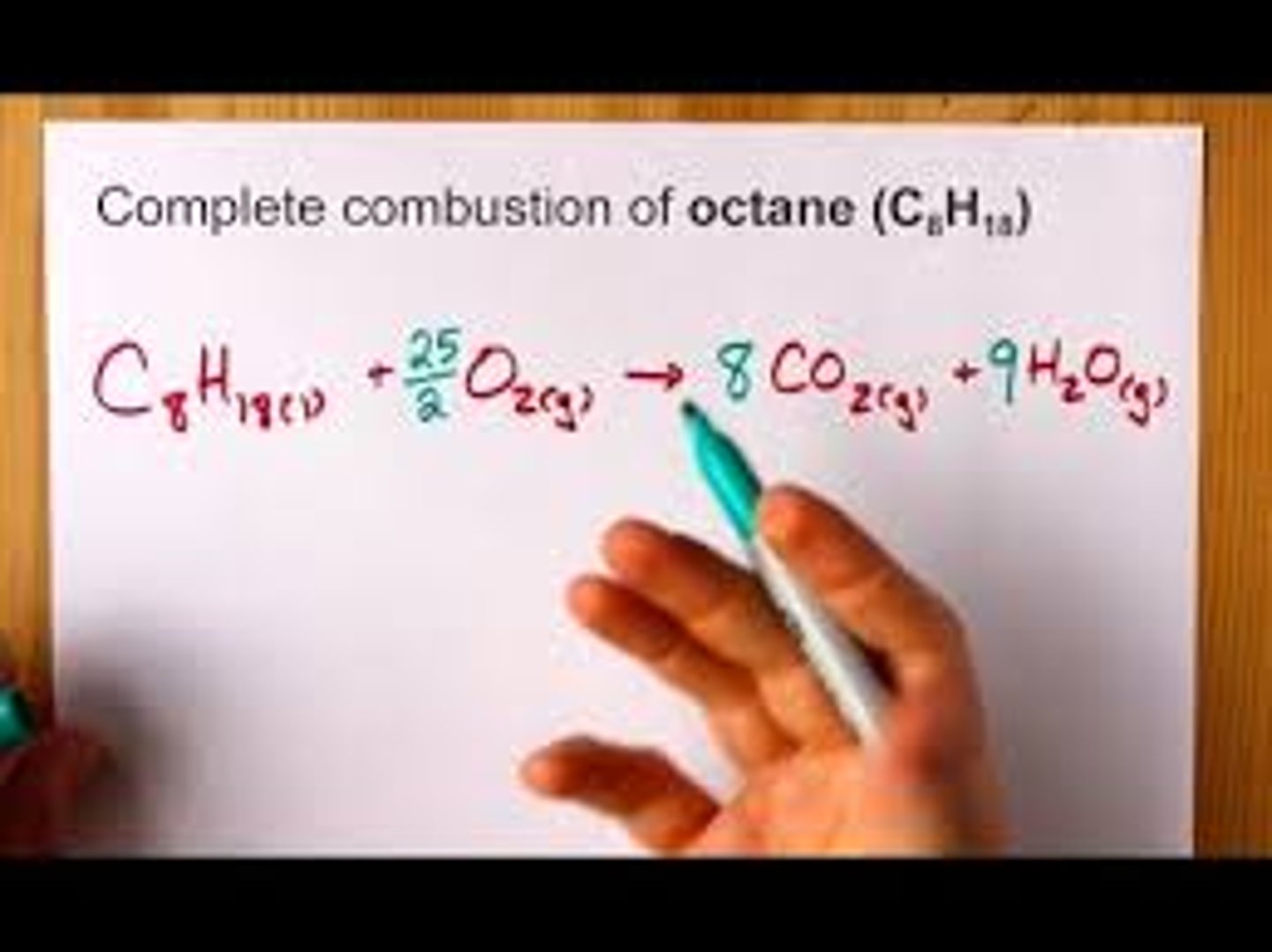
requires 25 g of O2
produces 16 g CO2
produces 18 g of H2O
complete combustion of octane C8H18
precipitate, gas or water
double-replacement reactions are driven by formation of

produces 8 CO2
complete combustion of butane C4H10
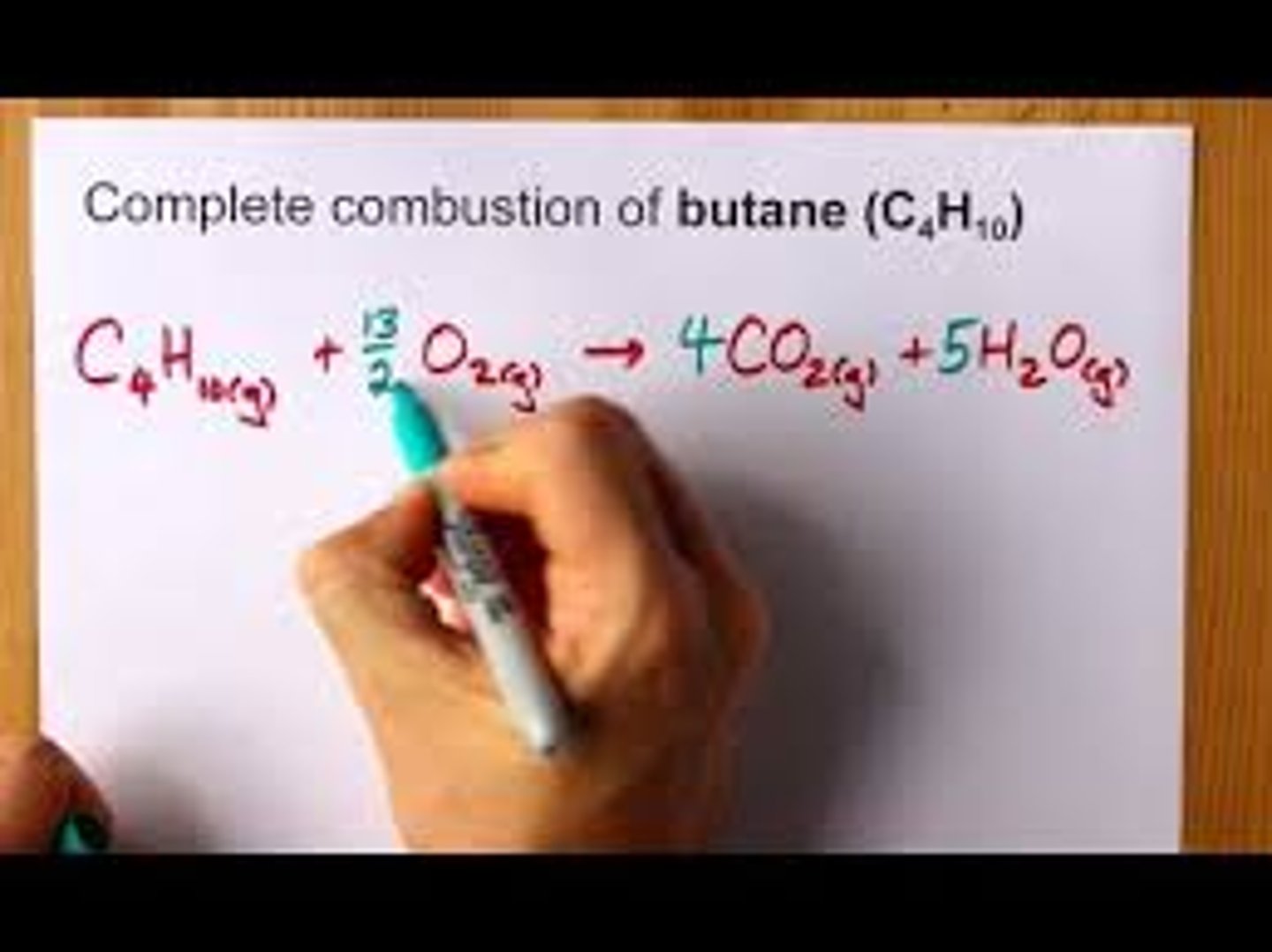
2 H2O are produced
To balance the double replacement reaction:
H2SO4 + NaOH -> Na2SO4 + __H2O
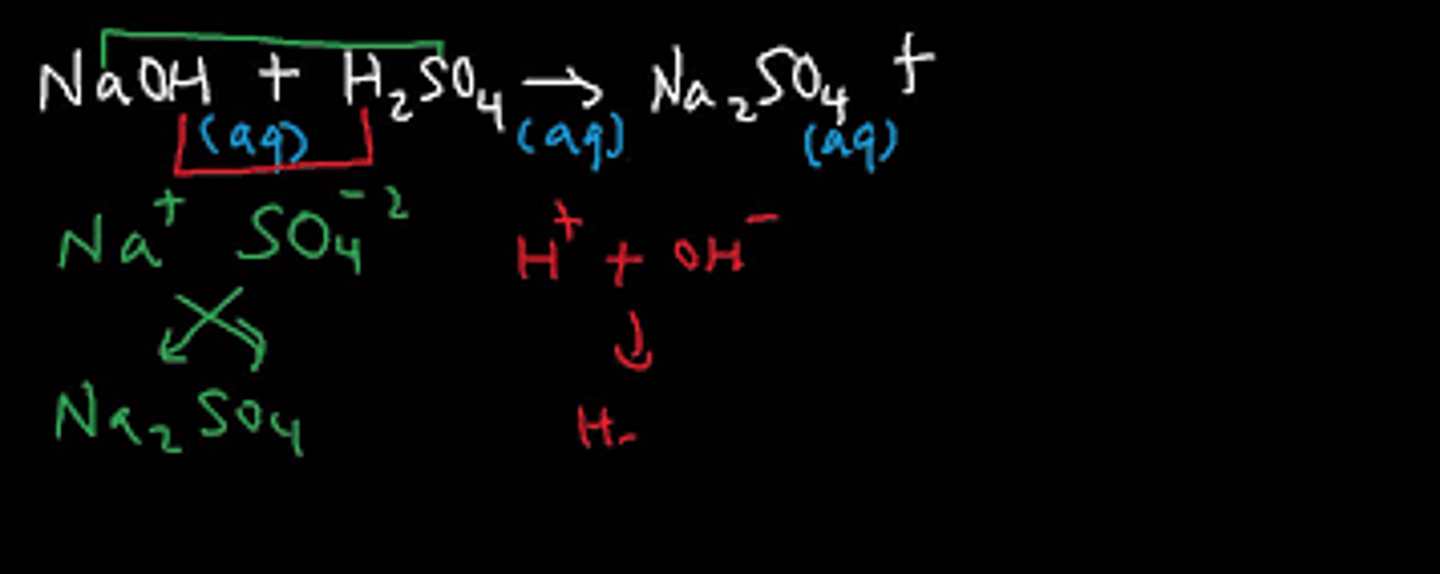
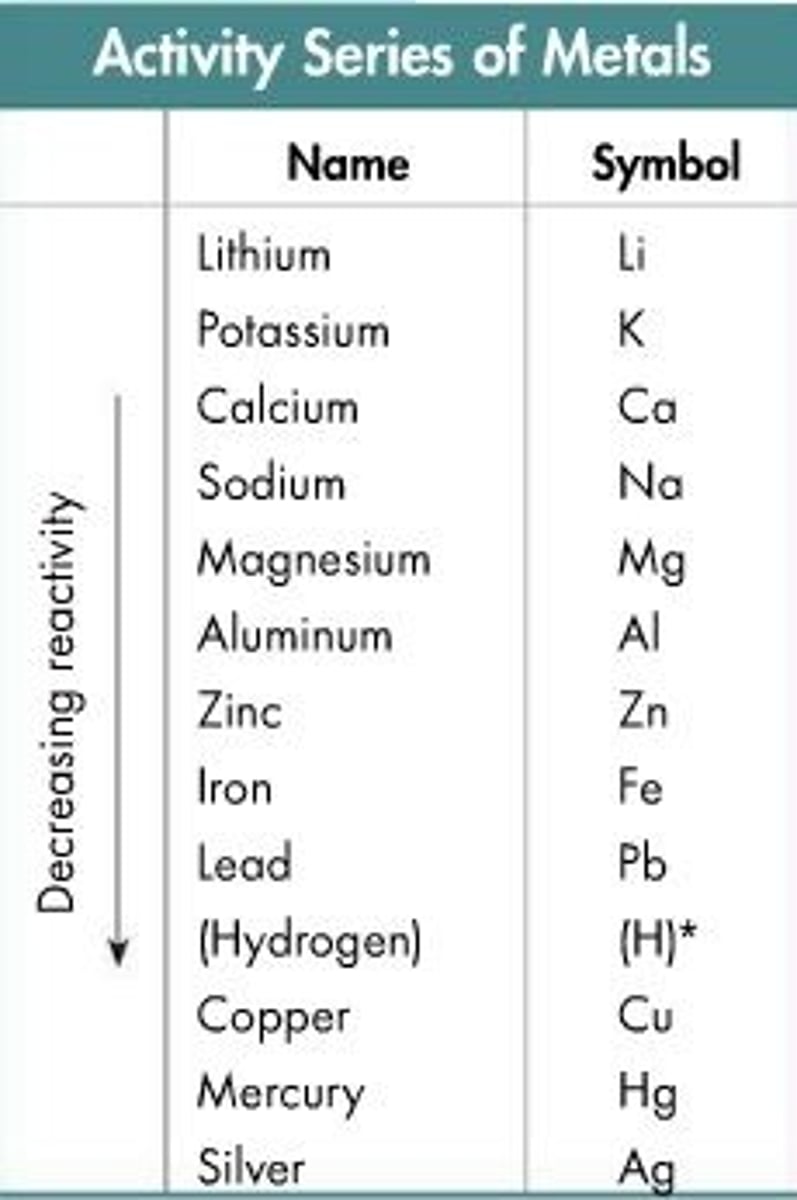
Zinc
Zn + HNO3 reaction occurs because of ___ metal's activity

Li and Cl are spectator ions
ZnCl2 + 2LiOH ->2LiCl + Zn(OH)2

CO2 + 2SO2
Cs2 +3O2->
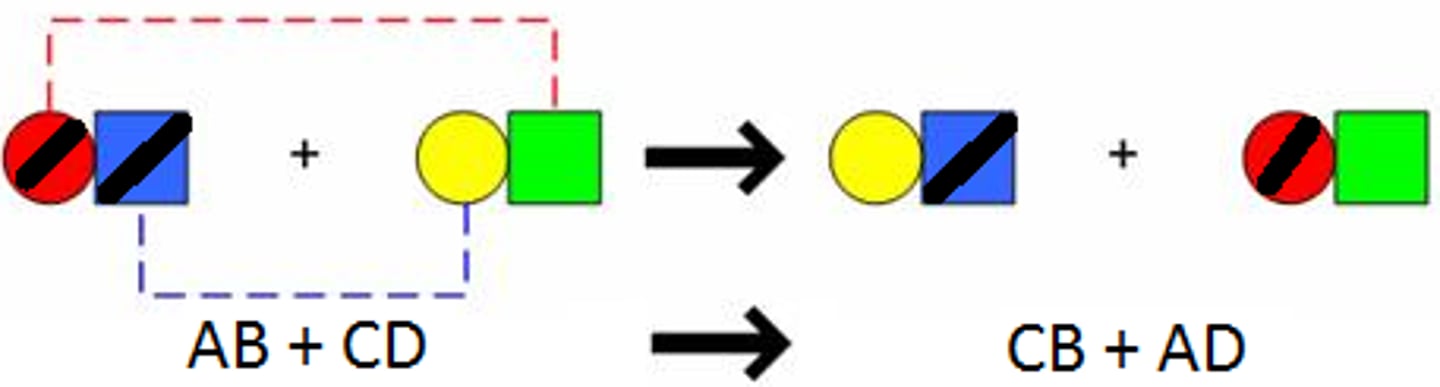
AB +CD ---> AD + CB
double replacement- two ionic compounds dissolved in water exchange ions to form 2 different ionic compounds, one of these is solid, water, or gas
E + FG -> EG + F
single replacement- more active metal replaces a less active metal in solution

2H2O
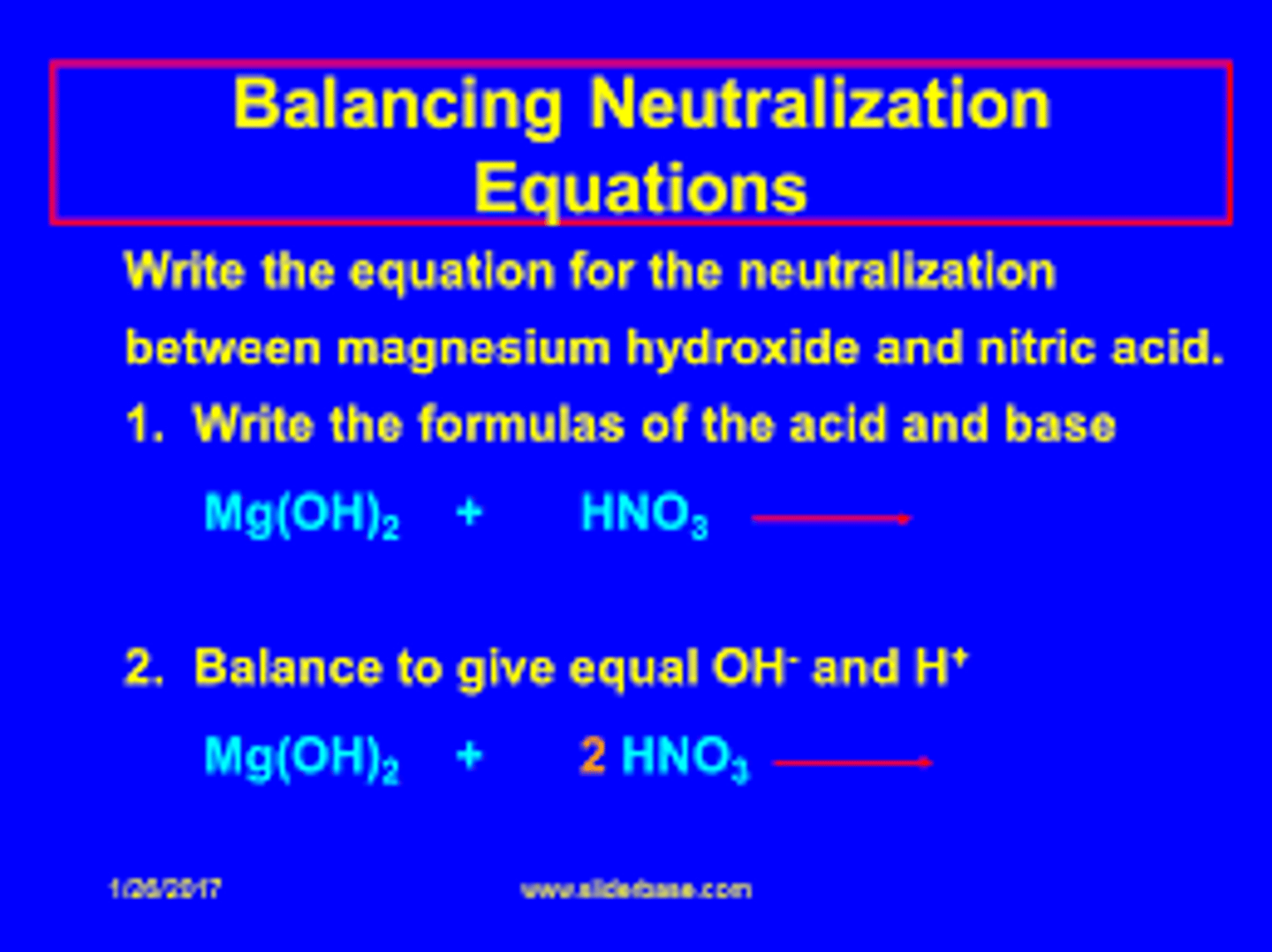
2HNO3 + Mg(OH)2 ->Mg(NO3)2 +_____
2LiOH
Combination reaction
Li2O + H2O forms_____________________

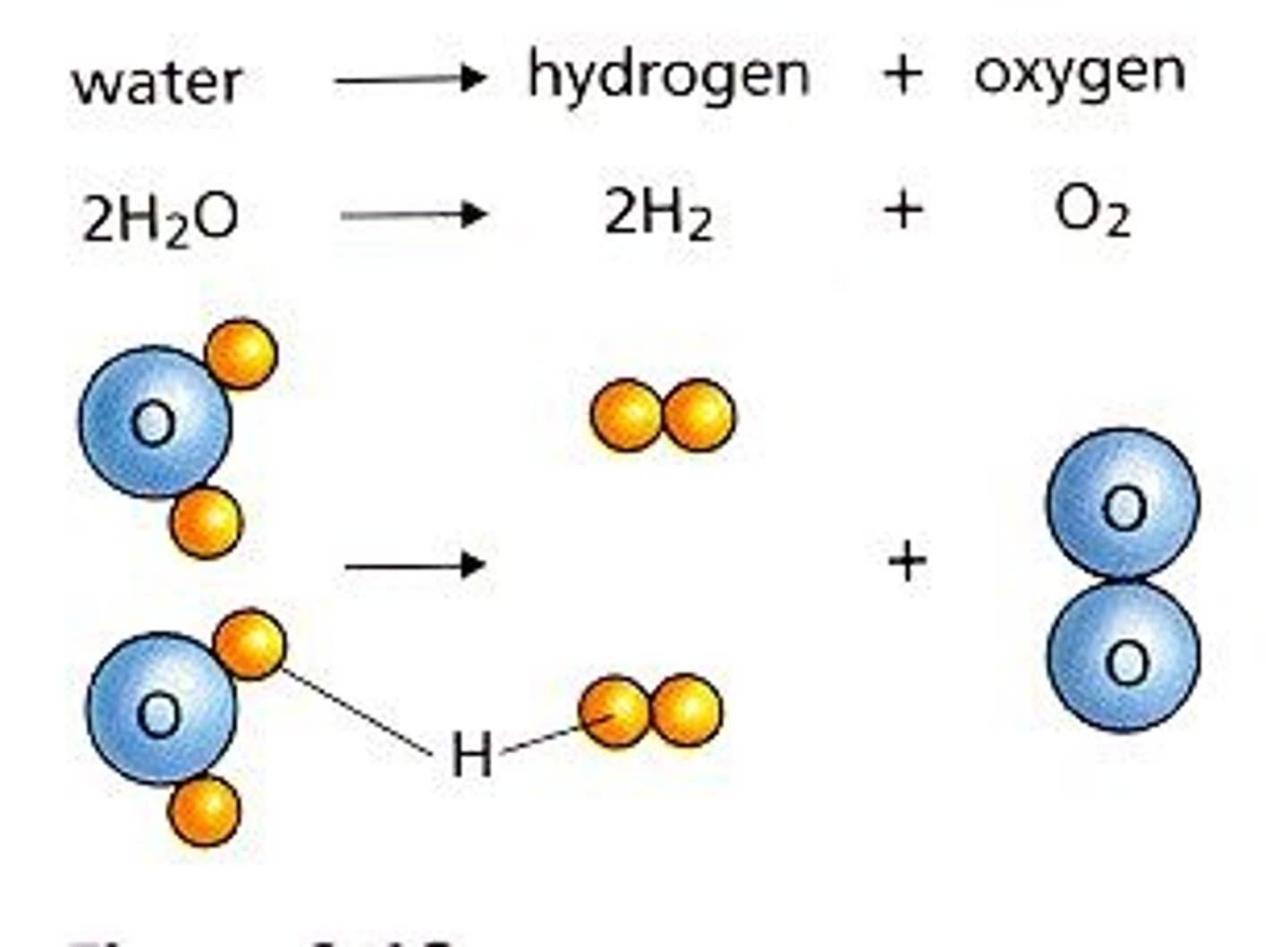
2H2 + O2
Decomposition reaction using electricity
2H20-> ___________________
