Local Anesthetic Agents (Week 3, Mod 7)
1/17
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What is the meaning of the word “anesthesia”?
Aesthesia – feeling or sensation
An – used to mean “absence of”
Anaesthesia – absence of feeling or sensation
Why is the use of local anesthetics more common than general anesthetics in large animals?
General anesthesia can be dangerous for large animals; being in lateral recumbency for so long, the dangers of coming out of it, etc.
What are the 7 main ways local anesthetics can be administered? Describe them.
1) Topical anaesthesia
To desensitise mucous membranes (oral, ocular, nasal etc)
To desensitise intact skin (EMLA cream)
2) Local infiltration
To desensitise dermal and subcutaneous tissues for minor surgery; small injections into multiple points
3) Instillation into a cavity or wound
Inter-pleural anaesthesia
Intra-articular anaesthesia (joints)
4) Intravenous regional anaesthesia (Bier’s block)
IV administration of lidocaine distal to a tourniquet
To desensitise distal limb e.g. digit amputation
5) Peripheral nerve blocks
Used diagnostically & therapeutically
Many possibilities e.g. Paravertebral, Intercostal, Brachial Plexus, & dental nerve blocks.
6) Epidural (extradural) block
To desensitise perineum, hindlimb &
caudal abdomen
7) Systemic administration
IV infusion of lidocaine in very
painful patients
What is a nociceptor? What is nociception?
Nociception - detection of noxious (painful) stimuli that actually or potentially causes damage to an organism
A nociceptor is then a specialized neuron that receives and processes painful stimuli
Describe the general pain pathway, starting from the stimulation of pain to the brain processing it.
1) Pain is received by the NOCICEPTOR
Generates an action potential (transduction)
2) Action potential travels up an AFFERENT fiber, which conducts an action potential to send to the CNS (transmission)
3) Processing occurs at the spinal cord, at the level of the dorsal horn (modulation)
4) The brain then receives the conscious experience of pain
In general, how do local anesthetics work? What process do they block?
Local anesthetics work by targeting the SODIUM ION CHANNELS on the membranes of neurons
Essentially blocks the ion channels and prevents sodium from rushing into the cell, keeping it from generating a membrane potential upon painful stimulation
What kind of drug is a local anesthetic? An acid? A base? Charged or uncharged? What does this mean in terms of distribution through the body? What is the active form of this drug?
Local anesthetics are WEAK, UNCHARGED BASES
This means that they can easily cross cell membranes to get into a cell
The active form of this drug is the CHARGED form (BH+)
Describe the mechanism of action of a local anesthetic… how does it block the sodium channels of the cell?
The drug is administered as a free, uncharged base, diffusing through the cell membrane easily (see image)
Is converted into its ACTIVE form (charged) in the cell, and physically blocks the sodium channels from WITHIN the cell
Specifically targets afferent nerve fiber, preventing the transmission of the pain stimulus by blocking the initiation and conduction of action potentials in the fiber.
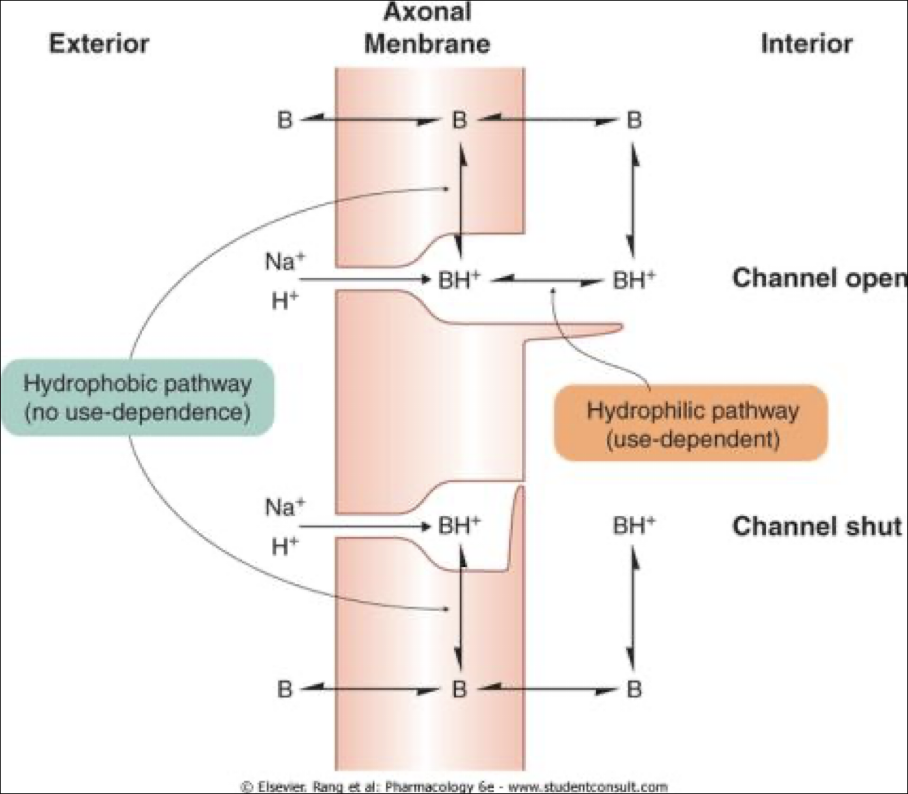
There are two different pathways by which the drug can become activated… what are they, and which one is use-dependent?
Hydrophobic pathway - becomes activated within the cell membrane and slips into the sodium channel from the side; can get into a channel whether its open or closed
Hydrophilic pathway - USE-DEPENDENT; drug becomes activated inside the cell, then enters the sodium channel from the interior; has to wait for the sodium channel to open
Describe the concept of a “use-dependent” drug… what does this mean?
Use dependence -> the more action potentials that are being fired from the receptor, the more rapidly and deeply the local anesthetic begins to work
This happens due to 2 factors:
The more action potentials that are fired, the more frequently channels are being opened. This allows more of the drug to get into the channels
Open and inactivate channels have a HIGHER AFFINITY for the drug; the more action potentials being fired the more those channels are in their open and inactive states, drawing more of the drug into the multiple channels
Essentially, use dependence is the concept of the local anesthetic having a stronger, more rapid effect, the more that action potentials are being fired
- This means that the more a patient is feeling, the quicker and harder the drug works
What 2 kinds of fibers are the most susceptible to the effects of local anesthetics? What does this mean in relation to the rest of the body?
Small myelinated fibers (A delta fibers) and small UNmyelinated fibers (C fibers)
Pain is most commonly received and carried by these fibers
Are more susceptible to local anesthetics than MOTOR nerve fibers
So lose sensation, but not function
What are the two different chemical structures that all local anesthetics consist of?
All localized anesthetics consist of a lipophilic aromatic ring and a basic hydrophilic amine side chain
linked either by an ESTHER bond or an AMIDE bond
How does the chemical structure of the local anesthetic assist in its distribution?
All localized anesthetics consist of a lipophilic aromatic ring and a basic hydrophilic amine side chain
This means that the drug is both lipid and water soluble
“Amphiphilic”
What is pH dependence? How does this effect the efficacy of the drug?
Local anesthetics are weak bases; they can be influenced and ionized at normal, physiological pH
Ionization INCREASES as pH falls; this can be an issue if there is any inflammation at the site of injection (environmental pH becomes more acidic, ionizing the drug before it can get into the target cells)
Inflammation therefore can DECREASE the efficacy of the drug, making it more difficult for the uncharged bases to pass through the membranes of the cells
Describe the pharmacokinetics of local anesthetics… how does it distribute through the body?
Speed of onset related to degree of ionisation (depends on acidity of environment)
Duration related to protein-binding (NOT plasma protein binding)
Binds to TISSUE PROTEINS instead; the greater the protein binding of the drug, the longer the drug will have an effect / the longer it will take to distribute through the body
potency related to lipid solubility
Other drugs may be added:
Bicarbonate (increases pH of environment)
Adrenaline (vasoconstricts)
Retains the drug at the site and exposes the area to the drug for longer
When should you NEVER use adrenaline??
When administering local anesthetic SYSTEMICALLY or as an EPIDURAL
How do ester-linked drugs and amide-linked drugs differ in their elimination from the body?
Ester-linked drugs
rapidly hydrolysed by non-specific cholinesterases
short half-lives
Amide-linked drugs
metabolised in the liver
longer half-lives (particularly in conjunction with adrenaline)
What are the 3 potential side effects in response to overdose or accidental IV administration? Which two are more common?
1) CNS: initial stimulation leading to convulsions
later depression leading to coma & death
2) CVS: myocardial contractility & heart rate fall
peripheral vasodilation
3) MISC: allergic reactions are rare (ester>amide)
methaemoglobinaemia?
Effects on the CNS and cardiovascular system are more common; ESPECIALLY if the patient already has pre-existing conditions