chemistry atoms (structure,history)
0.0(0)
Card Sorting
1/9
Earn XP
Description and Tags
1
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
1
New cards
element
made up of only one atom
2
New cards
mixture
(2 or more) different elements that aren’t chemically joined
3
New cards
compound
(1 or more) different elements chemically bonded together
4
New cards
atomic number
number of protons (same as number of electrons)
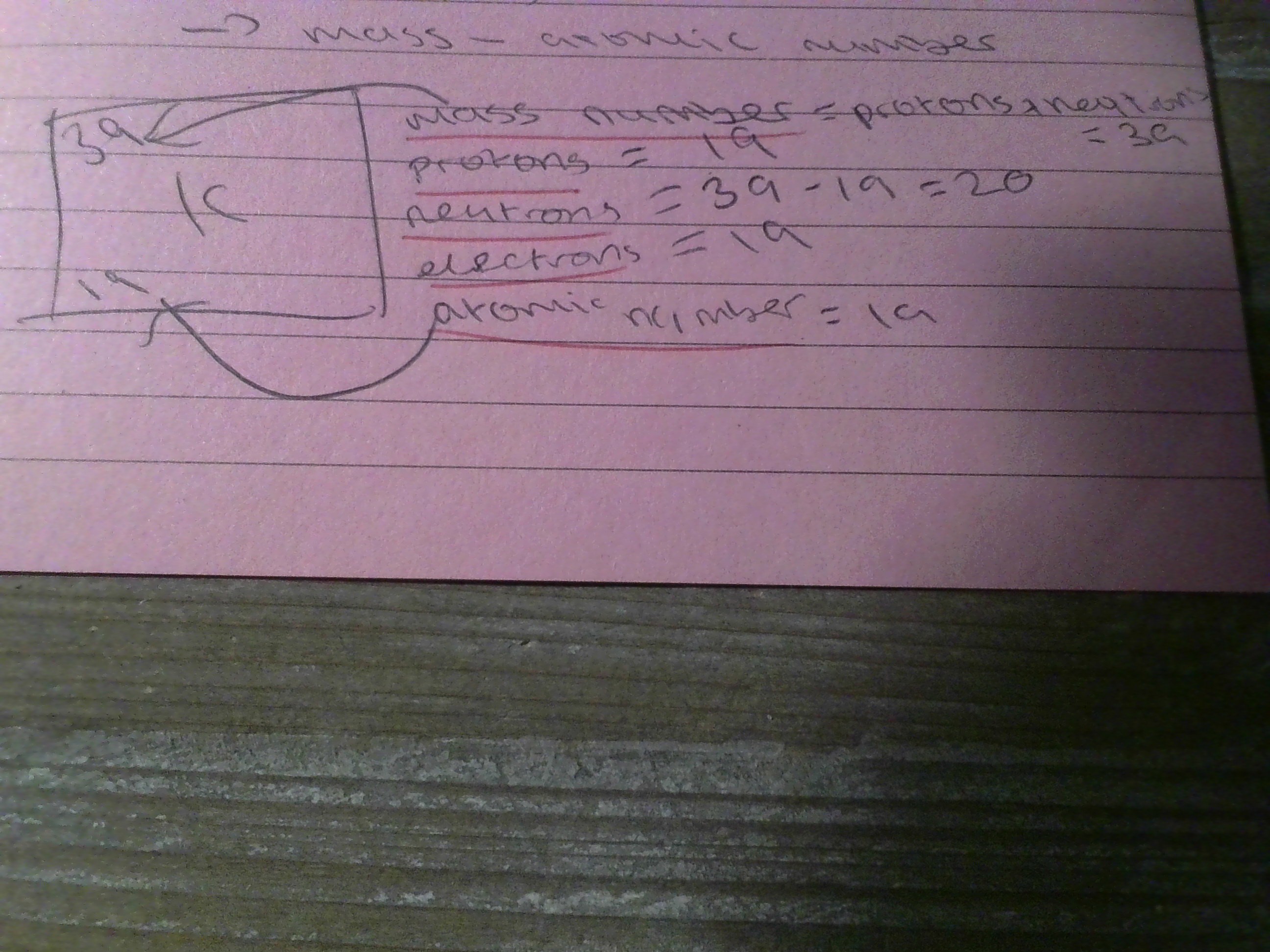
5
New cards
mass number
protons + neutrons
6
New cards
J.J THOMPSON
PLUM PUDDING MODEL:
1: no nucleus
2: scattered positive charge
3: no protons/neutrons
4: mass spread evenly throgouht atom
5: no electron shells, atoms have negatively charged electrons embedded within it

7
New cards
E.RUTHERFORD
NUCLEAR MODEL:
1: electrons in shells
2: positive charge in nucleus
3: mass in nucleus

8
New cards
JOHN DALTON
SOLID SPHERE MODEL
1803
1st SCIENTIST TO EXPLAIN ATOMS
9
New cards
NIELS BOHR
PLANETARY MODEL
1913
10
New cards
ERWIN S.
QUANTUM MODEL
1926