Unit 1: Lipids
1/136
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
137 Terms
Explain the physiological roles of cholesterol in the body, including its importance in cell membrane structure, hormone synthesis, and bile acid production.
Cholesterol is essential for cell membrane structure and fluidity, acts as a precursor for synthesizing steroid hormones like cortisol, aldosterone, and sex hormones (also used to produce Vitamin D), and is crucial for producing bile acids that aid in fat digestion.
It is integrated into cell membranes to modulate their fluidity and integrity, provides the building blocks for the body's steroid hormones (e.g., estrogens, testosterone), and is converted by the liver into bile acids that emulsify fats (break large fat globules into smaller particles that can be absorbed in the body) in the small intestine
Differentiate the 5 major lipoproteins (chylomicrons, VLDL, IDL LDL, HDL) based on their composition and primary function.
Lipoproteins are complex particles that transport fats and cholesterol in the blood
Chylomicrons: take fat from what we eat and transport it; transport dietary fat from the small intestine to the rest of the body
Highest lipid content (90% triglycerides) and lowest protein content
VLDL (very low-density lipoprotein cholesterol): delivers TGs to peripheral tissues (muscles, skin, organs, bones, blood vessels, lymphoid tissues- outside of the CNS (brain/spinal cord) from liver
LDL (low-density lipoprotein cholesterol): transports cholesterol to tissues "dominant form of atherogenic cholesterol"; deemed “bad cholesterol” because high levels are linked to atherosclerosis
HDL (high-density lipoprotein cholesterol): reverse cholesterol transport back to liver; good cholesterol; transports cholesterol from the tissues back to the liver
IDL (intermediate-density lipoprotein): transition between VLDL and LDL
Define dyslipidemia.
Dyslipidemia: abnormal levels of lipids in the blood
Elevated total cholesterol
Elevated LDL
Decreased HDL
Elevated TGs
Causes:
Genetics
Lifestyle factors (e.g., unhealthy diet, lack of exercise, smoking)
Medical conditions (e.g., diabetes, obesity, hypothyroidism)
Certain medications (e.g., birth control pills, corticosteroids)
Dyslipidemia is diagnosed through a blood test that measures lipid levels.
Treatment
Lifestyle changes (e.g., weight loss, exercise, smoking cessation)
Medications (e.g., statins, fibrates, niacin)
Describe common lipid abnormalities.
Total cholesterol
LDL-C
HDL-C
Triglycerides
lipid abnormalities are a primary risk factor for clinical atherosclerosis
coronary artery disease
cerebrovascular disease
peripheral artery disease
Abnormalities include
high levels of LDL-C: deposits cholesterol in arteries, forming plaques that causes blockages
high levels of TGs: a type of fat that can also contribute to artery plaque
low levels of HDL-C: high levels of HDL can help remove cholesterol from arteries
Metabolic syndrome: a cluster of conditions that includes high TGs, low HDL, high BP, and high blood sugar
Describe the common etiologies of dyslipidemia including primary and secondary.
Primary (familial): caused by inherited genetic abnormalities
Significant cholesterol elevations at an early age that accelerates atherosclerosis
Increases risk of premature clinical ASCVD
Types include: hypercholesterolemia, hypertriglyceridemia, combined hyperlipidemia, HDL metabolism disorders, lipoprotein excess, familial hypercholesterolemia
Secondary (acquired): triggered by environmental including lifestyle, diet, medications, or underlying diseases
Can occur independently or alongside genetic disorders
4D classification
Diet, drugs, disorders, diseases
Managed by addressing the underlying abnormality
Dietary causes:
Weight gain, excessive carb intake, high saturated fat intake, excessive alcohol use, anorexia
Medications:
Atypical antipsychotics, glucocorticoids, oral estrogen and progestin, diuretics, beta blockers, tacrolimus, cyclosporine
Diseases and disorders
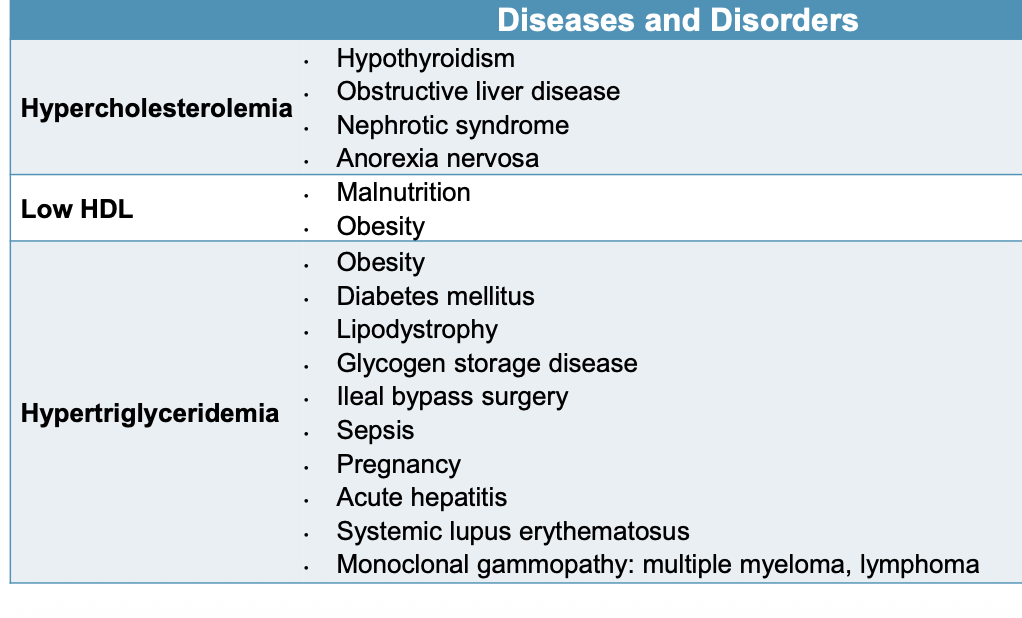
Hypercholesterolemia: hypothyroidism, liver disease, nephrotic syndrome, anorexia nervosa
Describe the exogenous pathway by which cholesterol is transported in the body via lipoproteins.
Exogenous Cholesterol Pathway - 20% outside of body
Lipid synthesis and transport
Dietary fats: arrive to small intestines as lipid droplets
Lipid droplets and cholesterol absorbed into intestinal cells
Intestinal cells make chylomicrons (large lipoprotein particles produced in the intestine that transport dietary fats, cholesterol, and fat-soluble vitamins throughout the body) (high in lipid content)
Fatty acids, cholesterol, apolipoproteins
Chylomicrons circulate fatty acids and triglycerides to tissues for energy
Chylomicrons remnants are transported to the liver and bind to LDL receptors to be removed from the blood
Describe the endogenous pathway by which cholesterol is transported in the body via lipoproteins.
Endogenous - 80% from inside body
Dietary glucose absorbed and delivered to the liver
Glycolysis occur: glucose --> pyruvate --> Acetyl -CoA
Acetyl - CoA --> cholesterol
HMG-CoA reductase
Acetyl-CoA also forms fatty acids and ultimately TGs
Explain the pathophysiology of atherosclerosis including:
Lipoprotein involvement in plaque formation
Composition and formation of atherosclerotic plaques
Definition and implications of vulnerable plaque
Consequences of plaque rupture
LDL accumulates in artery wall and triggers inflammatory response
macrophages engulf these lipids forming foam cells (filled with cholesterol lipids)
over time, the foam cells form into a plaque with a lipid core and a fibrous cap which can rupture
rupture of the plaque (collagen and other tissue factors) leads to a blood clot which can grow to completely block blood flow through the artery and potentially a heart attack or stroke
Describe lipoprotein synthesis and function
Lipoproteins are synthesized primarily in the liver and intestines as protein and lipid packages that transport cholesterol, TGs, and fatty acids throughout the bloodstream.
Liver makes 2 lipoproteins - VLDL & HDL (move lipids to tissues that need them for energy or building blocks)
VLDL (very LDL): transports fatty acids and TGs to muscles and adipose tissues
VLDL --> IDL --> LDL
Converted via lipase
LDL transports cholesterol to body tissues to carry out essential functions
LDL returns to the liver
Recycled in the golgi apparatus
Excreted into the bile (excess)
Reverse cholesterol transport (carry cholesterol back to the liver for disposal)
Empty HDL:
Secreted from the liver
Transported to the cells to remove excess cholesterol
Return to liver for recycling or excretion
picks up bad cholesterol and brings it back to liver
Explain how dyslipidemia contributes to atherosclerosis.
Dyslipidemia contributes to atherosclerosis through several mechanisms involving inflammation, oxidative stress, and the accumulation of lipoproteins within artery walls.
Excess LDL is the primary driver of plaque formation
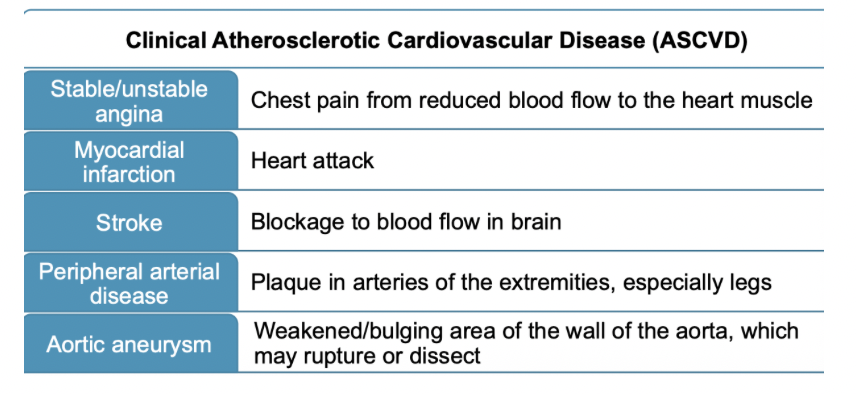
Discuss complications of atherosclerosis.
stable/unstable angina
chest pain from reduced blood flow to the heart muscle
myocardial infarction
heart attack
stroke
blockage to blood flow in brain
peripheral arterial disease
plaque in arteries of the extremities, especially legs
aortic aneurysm
weakened/bulging area of the wall of the aorta, which may rupture or dissect
Define clinical ASCVD
group of conditions that affect the arteries and increase the risk of CV events such as heart attacks and strokes
ASCVD, or Atherosclerotic Cardiovascular Disease, is caused by the buildup of plaque within the arteries, limiting the flow of blood to important organs
Identify examples of diagnoses classified as clinical ASCVD
Acute coronary syndrome (ACS)
range of conditions where blood flow to the heart is suddenly blocked, such as a heart attack
Myocardial infarction (MI)
a heart attack (blocked blood flow to the heart muscle)
specific type of ACS
Stroke
a cerebrovascular event caused by a blocked or ruptured blood vessel in the brain
Peripheral Artery Disease (PAD)
atherosclerosis in the arteries of the limbs
Angina
chest pain or discomfort that can occur with stable or unstable angina, a symptom of reduced blood flow to the heart
Revascularization Procedures
patients who have undergone procedures to restore blood flow to the heart or other arteries
Aortic Aneurysm
a bulge or swelling in the aorta, the body’s main artery
Describe how LDL-C lowering reduces the risk ASCVD.
by decreasing the formation and growth of atherosclerotic plaques in arteries
By keeping LDL-C levels low, fewer LDL particles become trapped in the artery wall, which slows the progression of atherosclerosis
positive relationship exists between elevated LDL-C and ASCVD risk
Risk of ASCVD rises more steeply with increasing LDL-C concentrations
LDL-C is the primary target of lipid-lowering strategies to reduce ASCVD risk
Treatment goal depends on level of individual risk of each patient
Define cardiovascular-kidney-metabolic (CKM) syndrome
a disorder connecting heart disease, kidney disease, type 2 diabetes, and obesity, where these conditions can worsen each other and lead to poor health outcomes
It is defined as a health disorder due to the interconnectedness of these four conditions, leading to an increased risk for heart attack, stroke, heart failure, and abnormal heart rhythms. The American Heart Association (AHA) recently defined this syndrome to emphasize the relationship between these conditions, which were previously often managed separately.
Introduce the concept of cardiovascular-kidney-metabolic (CKM) syndrome and its relevance to dyslipidemia.
a cluster of interconnected metabolic, kidney, and cardiovascular diseases that exacerbate each other.
It includes conditions like obesity, diabetes, chronic kidney disease (CKD), and cardiovascular disease (CVD), and is defined by the presence of at least two of these conditions.
Dyslipidemia, a key metabolic factor characterized by high triglycerides, low HDL cholesterol, or high blood pressure, is a significant component of CKM syndrome and contributes to its progression by driving inflammation, hemodynamic overload, and neurohormonal dysregulation
Describe the clinical implications of CKM syndrome, including its impact on ASCVD risk.
its most significant clinical implication is a dramatically heightened risk for atherosclerotic cardiovascular disease (ASCVD), heart failure, and premature death.
Interpret a fasting lipid panel.
dyslipidemia is diagnosed with a lipid panel
measures or estimates amount of “cholesterol” within lipoprotein
a lipid panel is a simple blood draw
most adults should have a lipid panel every 4 to 6 years
Presence of heart disease, diabetes, family history of high cholesterol, lowering require more frequent checks
Identify commonly used ASCVD risk calculators
ACC/AHA Pooled Cohort Equations (use this one)
AHA PREVENT calculator
Recognize limitations of ASCVD risk calculators.
Population-based limitations
racial and ethnic diversity (POOLED was on white and black individuals)
socioeconomic status: may overestimate risk in individuals with a higher socioeconomic status and healthier lifestyles and underestimate risk in those with lower socioeconomic status
older adults: not valid for adults over the age of 75
Incomplete inclusion of risk factors
family history
inflammatory and autoimmune conditions
specific biomarkers
Explain the rationale for ASCVD risk stratification (arranging in groups) in clinical practice.
The rationale for atherosclerotic cardiovascular disease (ASCVD) risk stratification is to classify patients into risk categories to guide the intensity of preventative therapy. The higher a patient's risk, the more intensive their treatment plan should be, ensuring that prevention efforts are targeted, effective, and efficient
Differentiate between primary and secondary prevention of ASCVD.
primary prevention (patient’s with no known clinical ASCVD, aim is to prevent or delay the onset of clinical ASCVD, shared decision-making around treatment goals and options, predict future risk of ASCVD events with a validated calculator)
assess patient’s ASCVD risk, if indicated
lifestyle modifications
medications
Active disease
lifestyle modifications
secondary prevention
lifestyle modifications
medications
Describe how lipid goals and treatment decisions differ based on risk group classification (e.g., low, borderline, intermediate, high)
Low risk (<5%)
Lifestyle is key: heart-healthy diet and regular exercise; statin is not usually recommended unless the patient has a very high LDL-C level (>/= 190 mg/dL)
Borderline risk (5% - < 7.5%)
personalized approach: a moderate-intensity statin may be considered especially if the patient has additional “risk-enhancing factors”; factors include a FH of premature ASCVD, chronic kidney disease, or elevated lipoprotein(a)
Intermediate risk (7.5% - < 20%)
moderate-intensity statin therapy: decision to initiate or intensity statin therapy is based on a discussion between the clinician and the patient; presence of risk-enhancing factors favors starting statin therapy
High risk (>/= 20% or other specific conditions)
high-intensity statin therapy: goal of reducing LDL-C by 50% or more; also includes individuals with severe hypercholesterolemia (LDL-C >/= 190 mg/dL) and most people with diabetes who are 40-75 years old
List ASCVD risk-enhancing factors.
family history of premature ASCVD
first degree relative: male <55 years, female <65 years
primary hypercholesterolemia
LDL-C of 160-189 mg/dL
persistently elevated primary hypertriglyceridemia (>/= 175 mg/dL)
Chronic Kidney Disease
eGFR 15-59 and not treated with dialysis or kidney transplantation
premature menopause (before age 40 years)
history of preeclampsia
south asian ancestry
Chronic inflammatory conditions
psoriasis, rheumatoid arthritis, HiV/AIDS
metabolic syndrome (have to have 3)
metabolic waist circumference (>40 inches males, > 35 inches females
elevated TGs >150 mg/dL
elevated BP >130/85 mmHg
elevated blood glucose levels (fasting >/=100 mg/dL)
low HDL-C of <40 mg/dL in men and <50 mg/dL in women
What is primary (familial) hypercholesterolemia?
caused by inherited genetic abnormalities
significant cholesterol elevations at an early age that accelerates atherosclerosis
increases risk of premature clinical ASCVD
What are types of primary hypercholesterolemia?
hypercholesterolemia, hypertriglyceridemia, combined hyperlipidemia, HDL metabolism disorders, lipoprotein excess, familial hypercholesterolemia
What is secondary (acquired) hypercholesterolemia?
triggered by environmental including lifestyle, diet, medications, or underlying diseases
Can occur independently or alongside genetic disorders
Classification (4D): Diet, Drugs, Disorders, Disease
managed by addressing the underlying abnormality
What are dietary causes for secondary hypercholesterolemia?
weight gain
excessive carb intake
high saturated fat intake
excessive alcohol use
anorexia
What medications cause secondary hypercholesterolemia?
increase both LDL-C and triglycerides
atypical antipsychotics
glucocorticoids
oral estrogen and progestin
diuretics
beta-blockers
tacrolimus
cyclosporine
What diseases and disorders cause secondary hypercholesterolemia?
Explain how ASCVD risk-enhancing factors (REFs) inform treatment decisions.
The presence of multiple REFs favors statin therapy for patients at intermediate or borderline risk
REFs allow clinicians to move beyond the general risk predicted by equations to provide a more personalized cardiovascular risk assessment.
Guide statin therapy: When an individual's 10-year risk falls into borderline (5−7.5%) or intermediate (7.5−20%) categories, REFs are used to help decide whether to initiate or intensify statin therapy.
Trigger more aggressive treatment: The presence of multiple or significant REFs can lead a clinician to recommend a more aggressive approach, such as starting a moderate- to high-intensity statin, even if the 10-year risk score is lower than typically recommended for that intensity.
Refine treatment decisions: REFs can be used to advocate for statin therapy in borderline-risk patients or for more intense therapy in intermediate-risk patients, particularly when the standard risk score may not fully capture the individual's risk.
Describe the role of coronary artery calcium (CAC) scoring in cardiovascular risk assessment.
Coronary artery calcium (CAC) scoring is a tool in cardiovascular risk assessment that measures calcified plaque in the coronary arteries using a CT scan to predict future heart events
Its role is to refine risk assessment for asymptomatic individuals, providing more accurate predictions than traditional risk factors alone by identifying those with low risk (CAC score of 0) and high risk (higher CAC scores)
It can guide clinical decisions, such as the need for statins and more aggressive lifestyle changes, particularly for those at intermediate risk.
What are the blood cholesterol patient management groups?
Primary prevention
LDL-C >/= 190 mg/dL
40-75 with diabetes
> 75 age group
40-75 age group
20-39 age group
0-19 age group
Secondary prevention
patients with ASCVD

Identify nonpharmacologic therapy for ASCVD-risk reduction.
How to treat lipoprotein disorders (primary prevention)?
therapeutic lifestyle changes are first-line for any lipoprotein disorder
Lipid lowering agents are chosen based on individual’s ASCVD risk
Identify nonpharmacologic therapy for ASCVD-risk reduction.
The cornerstone of ASCVD risk reduction
Recommended in all patients even when receiving lipid lowering therapy
12 week trial of lifestyle modifications
Recommended BEFORE lipid lowering therapy in patients without ASCVD or diabetes
Lifestyle modification ALONE is inappropriate for patients with established ASCVD or diabetes due to benefit of statin therapy
Weight and BMI should be determined at each visit
See chart for dietary patterns, physical activity, and tobacco

What are some recommendations to modify select lipid parameters (lower LDL-C, increase HDL-C, lower TGs)?
lower LDL-C
increase soluble fiber intake (goal 25 g/day)
fiber binds to cholesterol and bile in small intestines
reduces total and LDL-C
12 g/day of soluble fiber decrease LDL-C up to 12 mg/dL
fruits, vegetables, legumes, barley, oats/oat bran
phytosterol (2g/day) supplementation
found in various plant foods
can decrease LDL-C by 5-15%
vegetable oils, nuts/seeds, whole grains, fruits, legumes
increase HDL-C
increase physical activity
smoking cessation
lower triglycerides
lose weight (5%-10% body weight loss)
increase physical activity
abstain from alcohol
reduce intake of refined carbs and sugars
What is the MOA for statins (atorvastatin, rosuvastatin, simvastatin, lovastatin, pravastatin, fluvastatin, pitavastatin)?
inhibits HMG-CoA reductase
prevents conversion of HMG-CoA to mevalonate
it is the rate limiting step in cholesterol biosynthesis
reduced LDL synthesis
enhanced LDL catabolism (mediated through LDL receptors)
principal mechanisms for lipid-lowering effects
What is the first-line lipid lowering therapy?
“statins” - HMG-CoA reductase inhibitors
reduction in the risk of first CV event (primary prevention)
reduction in the risk of recurrent CV events (secondary prevention)
What are the effects of statins on the lipid profile (LDL, HDL, TG, VLDL)
reduces LDL-C by 20%-60%
increases HDL-C by 6%-12%
decreases TG by 10%-30%
reduces risk of ASCVD events
primary and secondary prevention
Explain why some statins require evening dosing.
Longer half life statins can be taken whenever (atorvastatin and rosuvastatin stay in the body for a full 24 hour cycle); pravastatin, simvastatin, lovastatin, fluvastatin need to be taken in the evening because of short half life (around 6 hours)
you produce the most cholesterol while you sleep
Describe how statins structure affects its absorption, distribution, metabolism, and excretion (ADME) profile.
atorvastatin, lovastatin, simvasatin
CYP3A4 enzyme metabolism
all are lipophilic (ability to dissolve in fats)
fluvastatin (CYP2C9); lipophilic
pitavastatin (moderate lipophilic), rosuvastatin (not lipophilic)
minimal CYP2C9
pravastatin
no CYP
not lipophilic
What are the ADRs of statins?
SAMs (statin-associated muscle symptoms)
reported by 10-25% of users; often a reason for discontinuation
myalgia (bilateral muscle achiness, weakness or cramps of larger muscle groups
rhabdomyolysis (rare): rapid breakdown of skeletal muscle
dark urine, AKI, N/V, confusion, coma, cardiac arrhythmias, electrolyte disturbances
elevations in serum transaminase levels
mild elevations in ALT
contraindication in decompensated cirrhosis or acute liver failure
new-onset diabetes
<1% increased risk of new-onset diabetes
common attributes of statin users who develop new-onset diabetes
higher statin doses and presence of other risk factors for diabetes
obesity
impaired fasting glucose
A1c >6%
metabolic syndrome
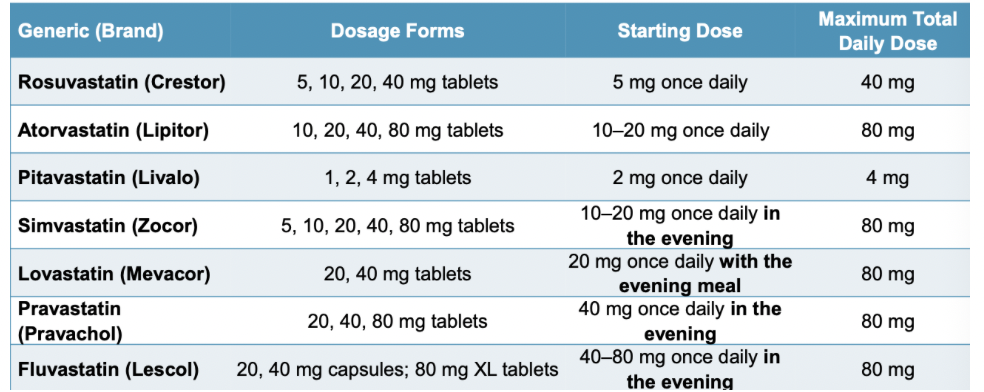
Which statins are high intensity therapies? What does that mean?
high intensity statins daily dose lowers LDL >/= 50%
rosuvastatin 20-40 mg
atorvastatin 40-80 mg
Which statins are moderate intensity therapies? What does that mean?
moderate intensity therapies daily dose lowers LDL from 30% to <50%
Rosuvastatin 5-10 mg
atorvastatin 10-20 mg
pitavastatin 2-4 mg
simvastatin 20-40 mg
lovastatin 40 mg
pravastatin 40-80 mg
fluvastatin XL 80 mg
fluvastatin 40 mg BID
Which statins are low intensity therapies? What does that mean?
low intensity statin therapy daily dose lowers LDL <30%
pitavastatin 1 mg
simvastatin 10 mg
lovastatin 20 mg
pravastatin 10-20 mg
fluvastatin 20-40 mg
Risk factors for SAMs
advanced age
female gender
low BMI
frequent heavy exercisers
increased serum statin concentrations due to drug-drug interactions
What are some medications that are CYP3A4 inhibitors? What does this do?
CYP3A4 inhibitors will increase serum statin concentrations and increase the risk for SAMS
CYP3A4 inhibitors: ketoconazole, itraconazole, ritonavir, clarithromycin, erythromycin, diltiazem & verapamil (Non-DHP CCB)
How to prevent SAMS?
for patients with multiple risk factors
lower starting doses
dose titration to the desired potency once the initial dose is tolerated
Explain statin intolerance.
5%-30% of patients will have satin intolerance
most common intolerance is SAMS
side effect that
resolves or improves after a dose decrease or discontinuation of statin therapy
after attempting at least two statins, with one at the lowest approved dose
complete inability to tolerate any statin dose
inability to tolerate dose required to achieve desired LDL-C level
nonstatin therapies may be considered in patients who fail multiple statins
What are nonstatin therapies?
ezetimibe
bile acid sequestrants
colestipol, colesevelam, cholestyramine
PCSK9 inhibitors
monoclonal antibodies (mAbs) molecules: evolocumab and alirocumab
small interfering ribonucleic acid (siRNA) molecule: Inclisiran
Bempedoic acid
When is ezetimibe preferred and why?
preferred adjunct therapy
reduces the risk of recurrent CV events in combo with statins (secondary prevention)
statin intolerance
What is the MOA for ezetimibe?
inhibits the NPC1L1 transporter protein in the small intestine from transporting cholesterol from the gut into the body; decreases the amount of cholesterol that reaches the liver so liver starts taking up LDL from the bloodstream
NPC1L1 protein absorbs dietary cholesterol and cholesterol from bile
prevents the absorption of cholesterol
leads to a reduction in circulating LDL-C
What are the lipid lowering effects of ezetimibe?
reduces LDL-C by 18%
higher LDL-C reductions achievable when used in combination with statin therapy (up to 25%)
What are ADRs for ezetimibe?
well tolerated
mild GI complaints (diarrhea)
myalgia and ALT elevations when used with statins
ezetimibe has not effects on the CYP450 enzyme
Role of ezetimibe in ADME?
Prodrug itself is converted into a more active form in the body. After absorption in the small intestine and liver, it is extensively converted into its pharmacologically active glucuronide metabolite( ezetimibe−glucuronide)
This metabolite is what exerts the cholesterol-lowering effects by inhibiting cholesterol absorption.
Conversion process:
Ezetimibe is converted into its glucuronide metabolite through a process called glucuronidation, mainly in the liver and small intestine.
Active metabolite:
The resulting ezetimibe-glucuronide is the primary active form of the drug circulating in the body and is responsible for the therapeutic effect.
Role of the prodrug:
The initial ezetimibe is the prodrug that, after being absorbed, is converted into the active compound to perform its function.
Pharmacokinetics:
The glucuronide metabolite is largely responsible for the prolonged plasma half-life of the drug.
What place in therapy are Bile Acid Sequestrants (BAS)?
first line during pregnancy
not systemically absorbed and pose no risk to the fetus
combination therapy with statins when desired LDL-C levels are not achieved with monotherapy
used when intolerance to statins and ezetimibe
What is MOA in Bile Acid Sequestrants (BAS)?
cholesterol is a precursor of bile acid
BAS bind bile acids in the intestinal lumen forming an insoluble complex that is excreted in the feces; prevents reabsorption of bile acids causing the liver to use more cholesterol to produce new bile acids. This process lowers cholesterol levels in the blood by reducing the amount of cholesterol in the liver which leads to an increase in LDL receptors on the liver surface
removes more LDL cholesterol from the circulation
stimulates hepatic synthesis of bile acids from cholesterol
increases cholesterol biosynthesis and the number of LDL receptors on hepatocyte membranes
more LDL receptors enhances the rate of LDL catabolism from plasma and lowers LDL-C levels
What are the lipid lowering effects of BAS?
reduces LDL-C by 13% to 20%
What are some BAS drugs?
Colesevelam (Welchol)
Colestipol (Colestid)
Cholestyramine (Question)
Do not administer in dry form; requires mixing with water or juice to create a slurry for oral administration
maintenance dose often based on patient tolerability
What are ADRs for BAS?
tablet formulations are generally better tolerated than resin powders
General GI complaints
constipation, bloating, epigastric fullness, nausea, flatulence
effects can be minimized by increasing fluid intake, increasing dietary bulk, and softeners
GI obstruction
Vitamin deficiencies due to impaired absorption of fat-soluble vitamins A, D, E, K
Hypertriglyceridemia
avoid when TG > 300 mg/dL
What are BAS drug-drug interactions?
reduced bioavailability of other drugs such as warfarin, levothyroxine, and phenytoin.
drug-drug interactions may be avoided by taking other mediations at least 1 hour before or 4 hours after BAS administration
What are PCSK9 Inhibitors?
PCSK9 drugs are a class of cholesterol-lowering medications that work by blocking the PCSK9 protein to help the liver remove more LDL (bad) cholesterol from the blood
used for individuals whose cholesterol is not controlled by diet and statins, including those with familial hypercholesterolemia
monoclonal antibody injections alirocumab (Praluent) and evolocumab (Repatha),
preferred in high-risk patients unable to achieve desired LDL-C levels with a statin + ezetimibe therapy
both drugs are FDA approved for use as monotherapy in patients with primary hyperlipidemia
small interfering RNA (siRNA) drug inclisiran (Leqvio)
reserved for patients unable to tolerate PCSK9 mAbs
FDA approved as additional therapy to be added to maximally tolerated statin therapy to further lower LDL-C
What is the MOA for PCSK9 inhibitors?
Target PCSK9 protein: PCSK9 drugs inhibit the PCSK9 protein, which normally breaks down LDL receptors on the liver.
Increase LDL receptors: By blocking PCSK9, the drugs allow more LDL receptors to remain active on the liver cells.
Lower LDL cholesterol: More active LDL receptors are able to capture and remove more LDL cholesterol from the bloodstream, resulting in significantly lower levels
Alirocumab and Evolocumab MOA
fully human monoclonal antibodies (mAbs) to PCSK9
prevents LDL receptors from being marked for degradation
more LDL-C is cleared from the blood lowering levels
Inclisiran MOA
small interfering RNA (siRNA) molecule complementary to the PCSK9 mRNA strand
binds to the PCSK9 mRNA strand resulting in degradation of PCSK9 mRNA leading to less PCSK9
allows for greater LDL receptor availability to remove LDL-C from circulation
What are the lipid lowering effects of PCSK9 drugs?
Alirocumab and evolocumab (injections given every two weeks)
reduces LDL-C (60%) when added to statin therapy
reduces recurrent CV events when added to statin therapy
Inclisiran (small interfering RNA - siRNA)
reduces LDL-C (40%-50%) when added to statin therapy
effect on CV event rates is currently being investigated
given SUBQ injection twice per year
What are the ADRs of PCSK9 drugs?
injection site reactions
minimize by allowing the medication to come to room temperature and icing the injection site
flulike symptoms
headache (evolocumab)
nasophyngitis (evolocumab)
urinary track infections (evolocumab)
What is bempedoic acid (Nexletol)?
an oral, non-statin medication used to lower low-density lipoprotein (LDL) cholesterol. It is particularly valuable for patients who cannot tolerate statins due to side effects like muscle pain.
patients take if unable to achieve desired LDL-C goal on statin + ezetimibe who prefer a noninjectable option
Bempedoic acid and ADME?
Bempedoic acid is a prodrug that is activated in the liver, not in muscle tissue, which is why it generally avoids the muscle-related side effects associated with statins
has a high plasma protein binding (99%)
activated in the liver by enzyme ACSVL1 to form an active coenzyme A ester, which inhibits ATP citrate lyase metabolism to form an inactive metabolite
absorbed in small intestines
metabolic pathway is hepatic (liver-based)
elimination is through metabolism in the liver and over 95% of the drug is excreted as metabolites
It is an adenosine triphosphate-citrate lyase (ACL) inhibitor
ACL is a cytoplasmic enzyme that generates acetylcholine coenzyme A
What is the MOA of bempedoic acid?
It works by inhibiting an enzyme called adenosine triphosphate-citrate lyase (ACL), which is involved in the cholesterol synthesis pathway.
By blocking this enzyme, it inhibits the conversion of Acetyl-CoA, reduces the amount of cholesterol produced in the liver, which leads to a corresponding increase in LDL receptors that clear "bad" LDL cholesterol from the bloodstream.
What are the lipid lowering effects of bempedoic acid?
reduces LDL-C by 15%-20%
bempedoic acid + ezetimibe results in a 36% reduction in LDL-C
What are the ADRs of bempedoic acid?
well tolerated
hyperuricemia
tendon rupture (rare)
What are fatty acids?
long chain of carbon atoms with carbons attached with carboxylic acid; saturated fatty acid has no double bonds; unsaturated fatty acids have double bonds (cis double bonds)
-trans fatty acids can stick to each other better which enabled melting points to be higher; bad for the heart like saturated fatty acids
-unsaturated fatty acids tend to be healthier (from plant sources)
-saturated fatty acids come from red meat
-a cell would want to utilize fatty acids for secondary energy source; cell uses carbohydrates to convert into glucose for energy; run out of fat – uses muscle (protein)
Why are fatty acids important?
they are a form of energy
play a role as structural components of cells (cell membranes)
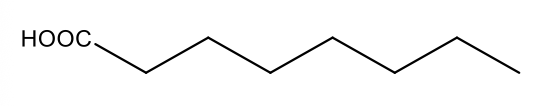
What are triglycerides?
glycerol is the backbone of a triglyceride (have 3 fatty acids); not water soluble- won’t go through blood easily; very lipophilic (want to clump together); simple lipids
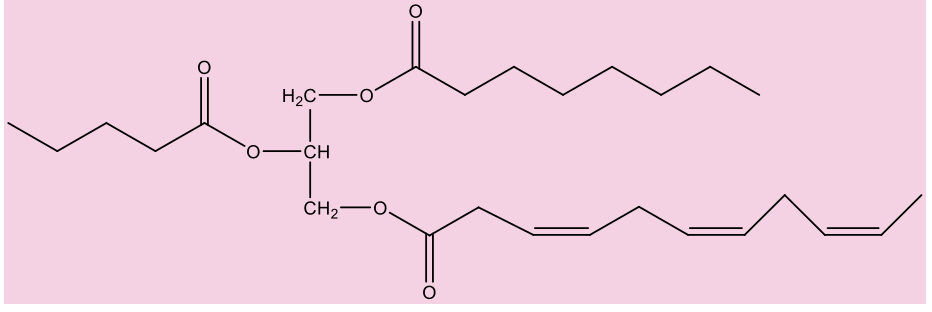
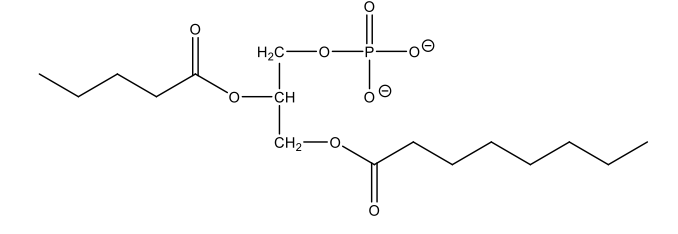
What is a phospholipid?
have glycerol backbone with 2 fatty acids (very lipophilic) and phosphate (PO4-) component (very polar); simple lipids – composed of fatty acids
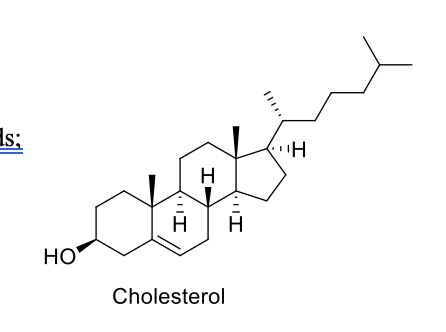
What are sterols?
lipophilic in nature and complex lipids; not made through fatty acids;
has one OH group
sterols have a fused four-ring core, while fatty acids consist of a long hydrocarbon chain with a carboxyl group at one end.
cholesterol is the most abundant sterol in animals
key component of animal cell membranes and a precursor for steroid hormones, vitamin D, and bile acids
Do sterols require transport?
Yes, sterols (lipids) require transport within cells and in the bloodstream because they are insoluble in water.
transport in the plasma as solubilized lipoproteins so necessary tissue gets the necessary lipids.
lipoproteins (carry cholesterol) consist of a central core of hydrophobic lipid encased in a more hydrophilic coat of polar phospholipids, free cholesterol, and associated proteins called apolipoproteins.
What is chylomicron?
Chylomicrons are the largest lipoprotein particles that transport dietary fats, including TGs, cholesterol, and fat-soluble vitamins from the small intestines to the rest of the body.
They are assembled in intestinal cells, then enter the lymphatic system before reaching the bloodstream, where they deliver their fatty acid contents to tissues.
Enzymes in the capillaries break down the triglycerides, and the remaining particles, called chylomicron remnants, are eventually taken up by the liver
What are chylomicrons composed of?
1% protein
88% TGs
3% cholesterol
transports dietary fats
What is VLDL (transporter) composed of?
10% protein
56% TGs
15% cholesterol
transports fats in the body (from liver)
What does LDL (bad cholesterol) consist of?
20% protein, 13% TGs, 48% cholesterol
transports fats in the body
What does HDL (good cholesterol) consist of?
50% protein
13% TGs
30% cholesterol
What are the two main sources of lipids?
exogenous (diet) - 20%
endogenous (hepatic biosynthesis) - 80%
What are the type of lipoproteins?
Chylomicron
VLDL
LDL
HDL
lipoproteins transport lipids through the bloodstream
What are TGs broken down by? Why are they broken down?
TG (stored form of fatty acid) is broken down by lipoprotein lipase (LPL) to form glycerol and fatty acids
TG are large, insoluble molecules that cannot be absorbed directly in the bloodstream.
Broken down to be absorbed in the body; Fatty acids can be taken up by cells
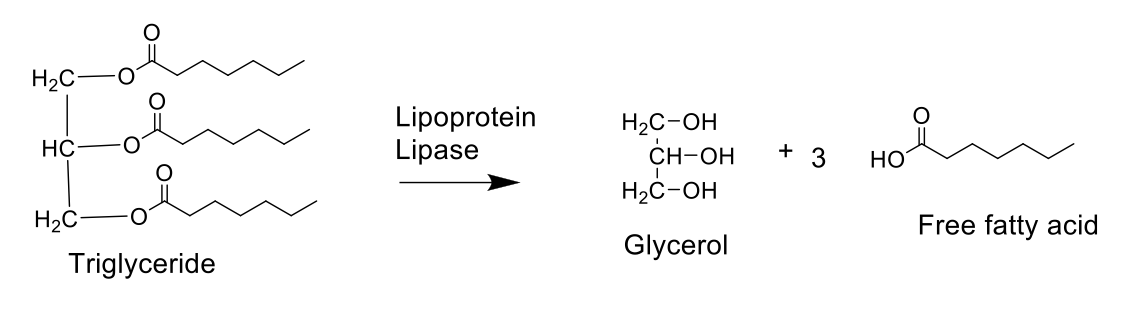
How do you get lipids from exogenous pathway?
The exogenous lipid process begins with dietary fats being digested in the small intestines by bile salts and pancreatic lipase.
These break down fats into absorbable components which are then reassembled into TGs inside the intestinal cells
These triglycerides, along with cholesterol and fat-soluble vitamins, are packaged into chylomicrons, which are secreted into the lymphatic system before entering the bloodstream to be delivered to tissues (muscle) for energy or storage (adipose tissue)
After binding to lipoproteins, ApoC-II acts as a crucial cofactor for lipoprotein lipase (LPL). LPL is anchored to the interior surface of capillaries in tissues that require fatty acids, such as adipose tissue and muscle.
It is on the surface of blood vessels which breaks down chylomicrons, releasing lipids for use by cells.
The ApoC-II present on the chylomicron interacts with and activates LPL, triggering the enzyme to begin hydrolyzing the triglycerides (TGs) contained within the core of the chylomicron.
After releasing most of its triglycerides, the chylomicron shrinks and becomes a cholesterol-rich chylomicron remnant.
ApoE: The chylomicron remnant is recognized and taken up by the liver. This clearance process is mediated by ApoE on the remnant, which binds to specific receptors (like the LDL receptor) on liver cells.
The liver then processes the remnants, and the components can be recycled or converted into bile acids
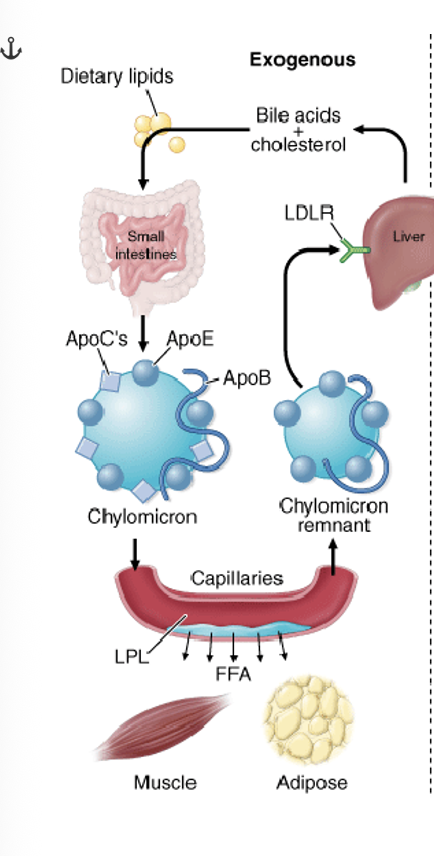
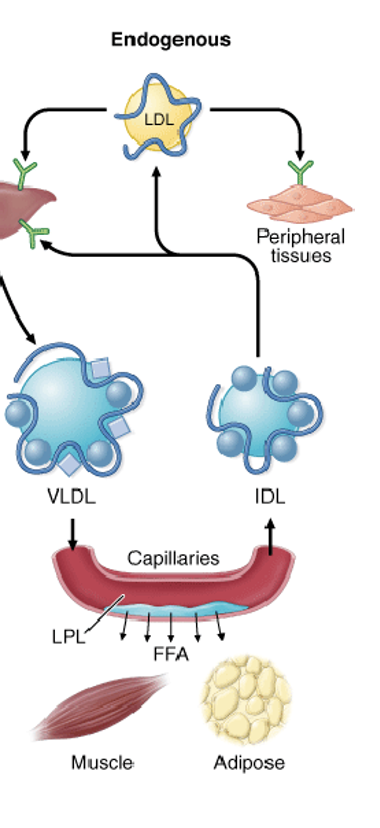
What is the endogenous pathway?
Formation:
The liver packages synthesized lipids (triglycerides, cholesterol) and apoB-100 into VLDL particles.
Modification:
VLDL is released into the bloodstream, where it is matured by receiving apolipoproteins like apoC-II and apoE from high-density lipoproteins (HDL).
Lipolysis:
ApoC-II activates lipoprotein lipase (LPL), which breaks down VLDL's triglycerides into free fatty acids for tissue use.
Remnant formation:
As VLDL loses triglycerides, it becomes an intermediate-density lipoprotein (IDL).
Clearance:
IDL can be taken up by the liver via the apoE receptor or further converted into low-density lipoprotein (LDL) by hepatic lipase
What is the primary site of cholesterol synthesis in the body?
Liver
What do LDL receptors do?
Interaction leads to uptake of the whole LDL particle;
it is broken down from LDL particle into its components and returned to LDL receptor to surface
Blood Cholesterol Patient Management Groups for primary prevention of ASCVD chart
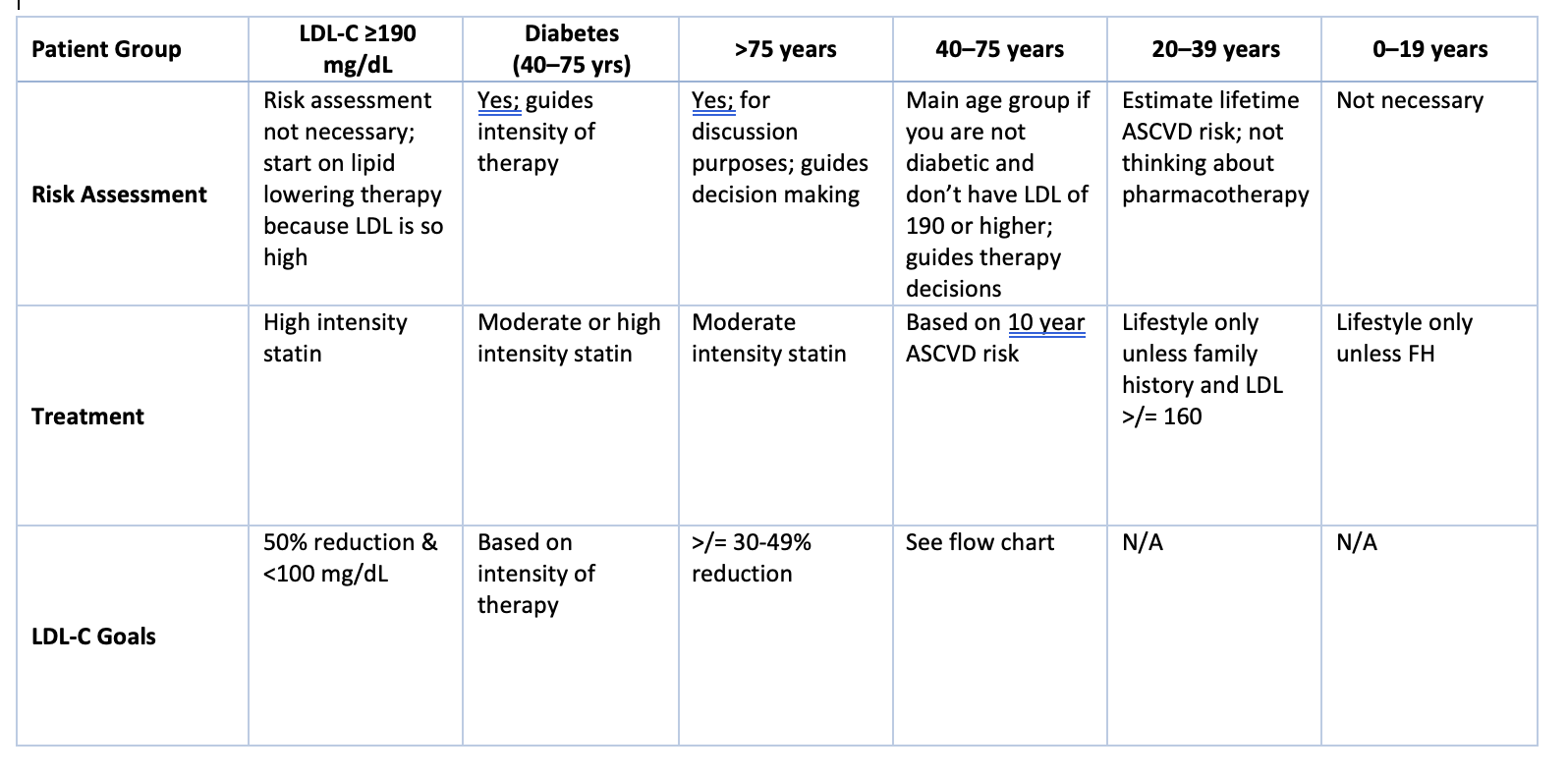
What are the treatment plans for primary prevention (severe primary hypercholesterolemia - LDL-C >/= 190)?
Do not calculate 10 year ASCVD risk
Begin high intensity statin therapy
Achieve goal of 50% reduction in LDL-C
Goal LDL-C <100 mg/dL
If goal is not achieved
Add ezetimibe FIRST then a PCSK9 inhibitor
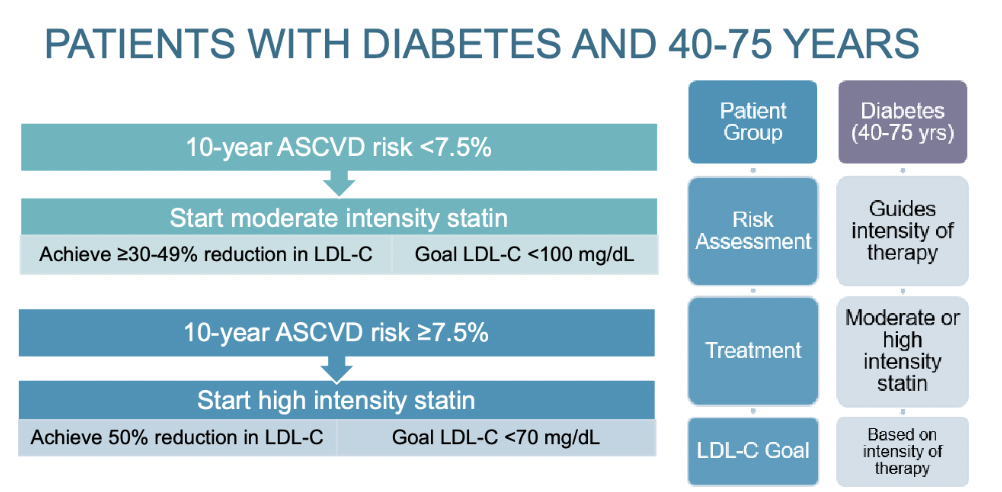
Treatment plan for 40-75 year olds with diabetes?
Diabetes is a major risk factor for ASCVD
Leading cause of death in persons with diabetes is ASCVD
Persons with diabetes are at greater risk of morbidity and mortality following an ASCVD event than those without diabetes
10 Year ASCVD risk score is used to determine appropriate statin therapy
less than 7.5% - start moderate intensity statin; goals: achieve >/= 30-49% reduction in LDL-C and LDL-C <100 mg/dL
greater than or equal to 7.5% - start high intensity statin; goals: achieve 50% reduction in LDL-C and LDL-C <70 mg/dL
If the goal is not achieved
Increased to high intensity statin (if not already on)
Can add ezetimibe FIRST then a PCSK9 inhibitor
What is the treatment plan for patients >75 years old?
Most ASCVD events occur in the 6th and 7th decade of life
Older patients respond to therapy as well as younger patients
The gain in life expectancy may be small
Depends on age at the start of treatment and the magnitude of LDL-C reduction
Benefits of statins for primary prevention is debated
Patients >75 is poorly represented in clinical trials
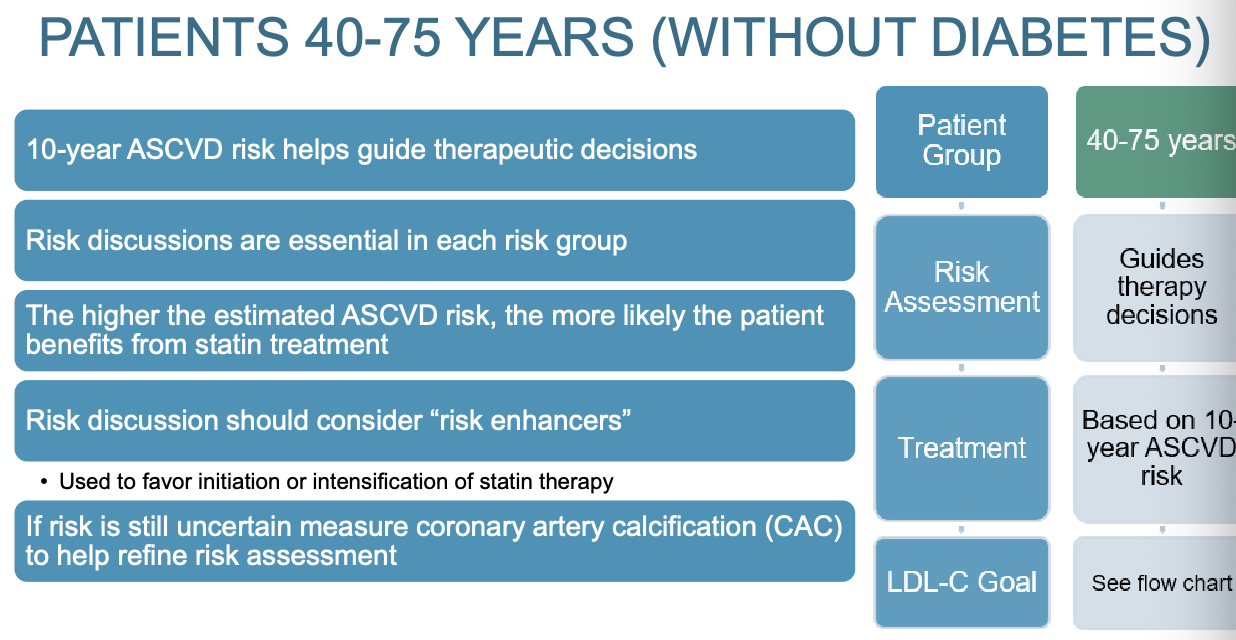
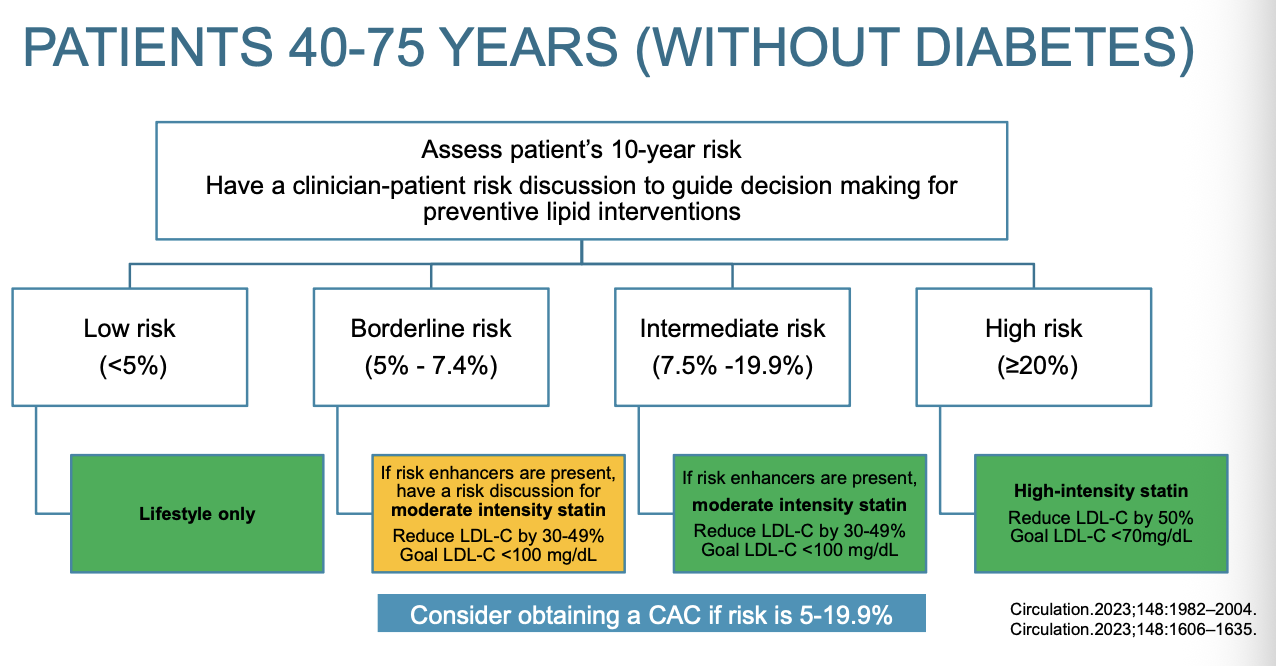
What is the treatment plan for patients 40-75 years old without diabetes?
Flow chart for patients 40-75 years old without diabetes
Yellow means to proceed with caution
Low risk: Lifestyle only
Borderline risk: lifestyle; most discussion with patient; possible moderate intensity statin; reduce LDL-C by 30-49% and goal LDL-C <100 mg/dL; considering getting CAC score
Intermediate risk: same as borderline but more discussion in borderline
High risk: high intensity therapy; reduce LDL-C by 50%; Goal LDL-C <70 mg/dL
What should a risk discussion include?
Clinicians and patients should engage in a risk discussion:
Determine if ASCVD risk factors have been addressed
Evaluate if lifestyle modifications have been implemented
Review potential for adverse effects and DDI with lipid lowering therapy
Consider patient preferences
Consideration given to "risk enhancers" and CAC scoring
Used to favor initiation or intensification of statin therapy
What are ASCVD risk enhancers?
Family history of premature ASCVD
First degree relative: Male <55 years, female < 65 years
Primary hypercholesterolemia
LDL-C of 160-189 mg/dL
Persistently elevated primary hypertriglyceridemia (>/= 175 md/dL)
CKD
eGFR < 60
ASCVD event reduction is less robust than patients without CKD
Moderate intensity statins are preferred to minimize the risk of adverse effects
Ezetimibe may also be used in combination with statin therapy
Current guidelines do not advocate for routine use of other nonstatin therapies
Premature menopause (before age 40 years)
History of preeclampsia
South Asian ancestry
Chronic inflammatory conditions
Psoriasis, rheumatoid arthritis, HIV/AIDS
Metabolic syndrome (need 3 to make diagnosis)
Increased waist circumference
>40 inches males; >35 inches females
Elevated triglycerides >150 mg/dL
Elevated blood pressure (>130/85 mmHg)
Elevated blood glucose (fasting >/=100 mg/dL)
Low HDL-C of <40 mg/dL in men or <50 mg/dL in women
What is CAC?
CAC (Coronary Artery Calcification)
Predicts future ASCVD events
Atherosclerotic plaques consists of cholesterol, fat, calcium, fibrin, and other cellular waster products
CAC = 0; delay or withhold statin therapy Unless DM, and those with strong family history of premature ASCVD
CAC = 1-99; favors statin therapy
CAC >/= 100; statin therapy is indicated
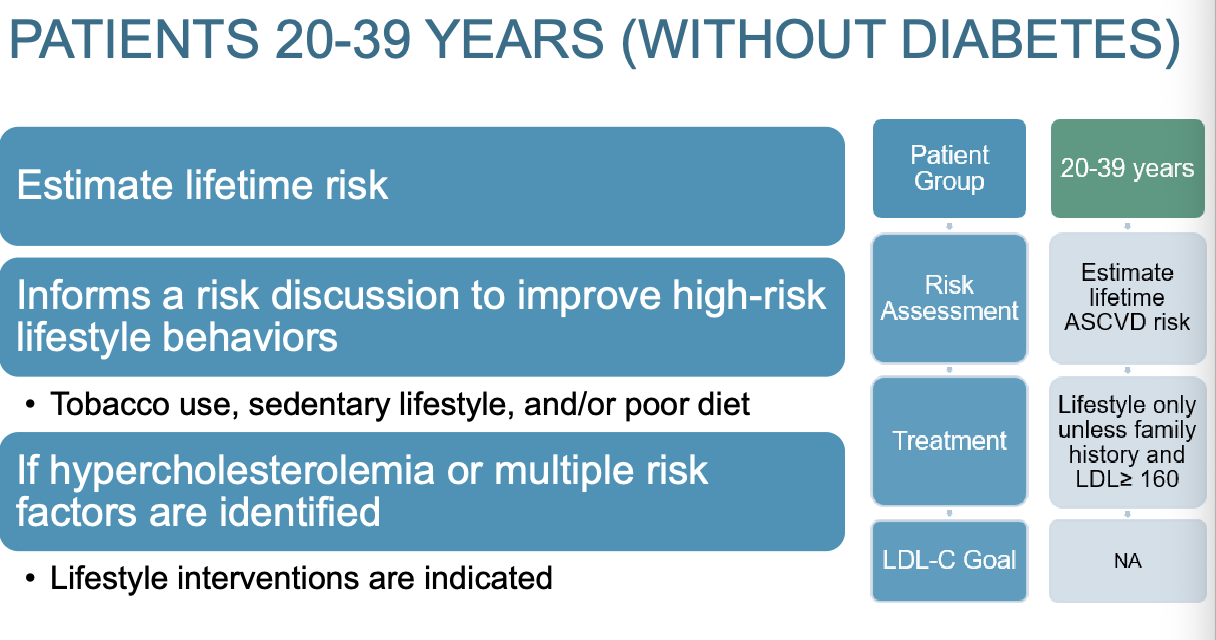
What is treatment for patients 20-39 years old without diabetes?