every topic done in year 9
1/123
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
124 Terms
what are the uses of nanoparticles?
- catalysts
- in nanomedicine
- for their antibacterial properties
why are nanoparticles used as catalysts?
when using things as catalysts, its the exposed surface area thats important. so that means we need much less of a material made of nanoparticles than we would a material made out of bigger particles because nanoparticles have a HUGE SA:VOL ratio.
why are nanoparticles used in nanomedicine?
their small size and high sa:vol ratio enables enable targeted drug delivery. an example is the use of fullerenes to deliver drugs around the body.
why are nanoparticles used in electrical currents?
some nanoparticles can conduct electricity so theoretically we could make tiny computer chips.
why are nanoparticles used for their antibacterial properties?
for example, silver nanoparticles have antibacterial properties. for example, we can infuse them into surgical masks to kill bacteria.
what are the issues of nanoparticles?
nanoparticles are a relatively new thing, so its effects on our bodies aren't fully understood yet.
what is the diameter of coarse particles (PM10)?
between 1 x 10^-5 m and 2.5 x 10^-6 m
what is the diameter of fine particles (PM2.5)?
between 1 x 10^-7 and 2.5 x 10^-6
what is the diameter of nanoparticles?
between 1 x 10^-9 and 1 x 10^-7
how much is 1 mm (millimetre) in metres?
1 x 10^-3
how much is 1 µm (micrometres) in metres?
1 x 10^-6
how much is 1 nm (nanometres) in metres?
1 x 10^-9
how do you find the surface area of an object?
area of one face x number of sides
how do you find the volume of an object?
length x width x height
how do you find the surface area : volume ratio of an object?
surface area x volume
what is an alloy?
a mixture of two or more elements, at least one of which is a metal.
how are alloys harder than pure metals?
in an alloy, the different sizes of atoms distorts the layers. This makes it more difficult for the layers to slide over each other.
what are fullerenes?
a group of carbon allotropes which consist of molecules that form hollow tubes or spheres.
buckminsterfullerene (c60) contains __ carbon atoms arranged in a ______ sphere
60, hollow
the carbon atom in buckminsterfullerene form rings with either _ or _ carbon atoms.
6,5
name all 3 uses of buckminsterfullerene.
- used to deliver drugs (pharmaceuticals) into the body
- used as lubricant in machines
- used as catalyst to speed up reactions
carbon nanotubes are fullerenes _________ (multiple words)
shaped into long cylinders with a relatively small diameters
carbon nanotubes have rings formed from _ carbon atoms
6
name all three properties of carbon nanotubes.
- high tensile strength (meaning you can apply a great deal of stretching force to it before it breaks)
- good conductors of electricity
- good conductors of heat
what are the uses of carbon nanotubes (only one use) ?
they are used to reinforce materials (like high end tennis rackets)
what is graphene?
a single layer of graphite
how thick is graphene?
one atom thick
name all 3 properties of graphene (similar to graphite)
- good conductor of electricity
- extremely strong
- high melting and boiling points
why is graphene a good conductor of electricity?
- graphene has delocalised electrons
- these delocalised electrons can move through the graphene, carrying electrical charge
how does graphene has a high melting and boiling point?
- graphene has a large number of strong covalent bonds, and these covalent bonds require a great deal of energy to break.
what are the properties of graphite?
- graphite is soft and slippery
- graphite has a very high melting and boiling point
why does graphite have a very high melting/boiling point?
because graphite contains a large number of strong covalent bonds. So, if we want to melt graphite, then we would need to break those covalent bonds. This takes a great deal of energy.
Why is graphite soft and slippery?
Weak forces between the layers allow them to slide over each other easily.
Why does graphite conduct electricity?
Each carbon atom has one delocalised electron that is free to move and carry charge.
graphite is formed from ______________ (multiple words)
layers of carbon atoms arranged in hexagonal rings.
How many covalent bonds does each carbon atom form in graphite?
three covalent bonds
"Describe the structure and bonding in graphite and explain how this relates to its properties."
-Graphite is made of carbon atoms arranged in layers of hexagons.
-Each carbon atom forms three strong covalent bonds with other carbon atoms.
-The fourth electron from each atom is delocalised and free to move throughout the structure.
-The layers are held together by weak forces, allowing them to slide over each other easily.
-This makes graphite soft and slippery.
-The delocalised electrons can move and carry charge, so graphite conducts electricity.
What state are giant covalent molecules in room temperature?
Solid
Giant covalent molecules have ______ melting and boiling points
high
Why does giant covalent molecules have high melting and boiling point?
Because they have millions of strong covalent bonds (in order to melt or boil these substances, we have to break all of these covalent bonds, which requires a lot of energy)
Diamond is formed by
huge number of carbon atoms joined by covalent bonds
In a diamond, each carbon atom forms
4 strong covalent bonds
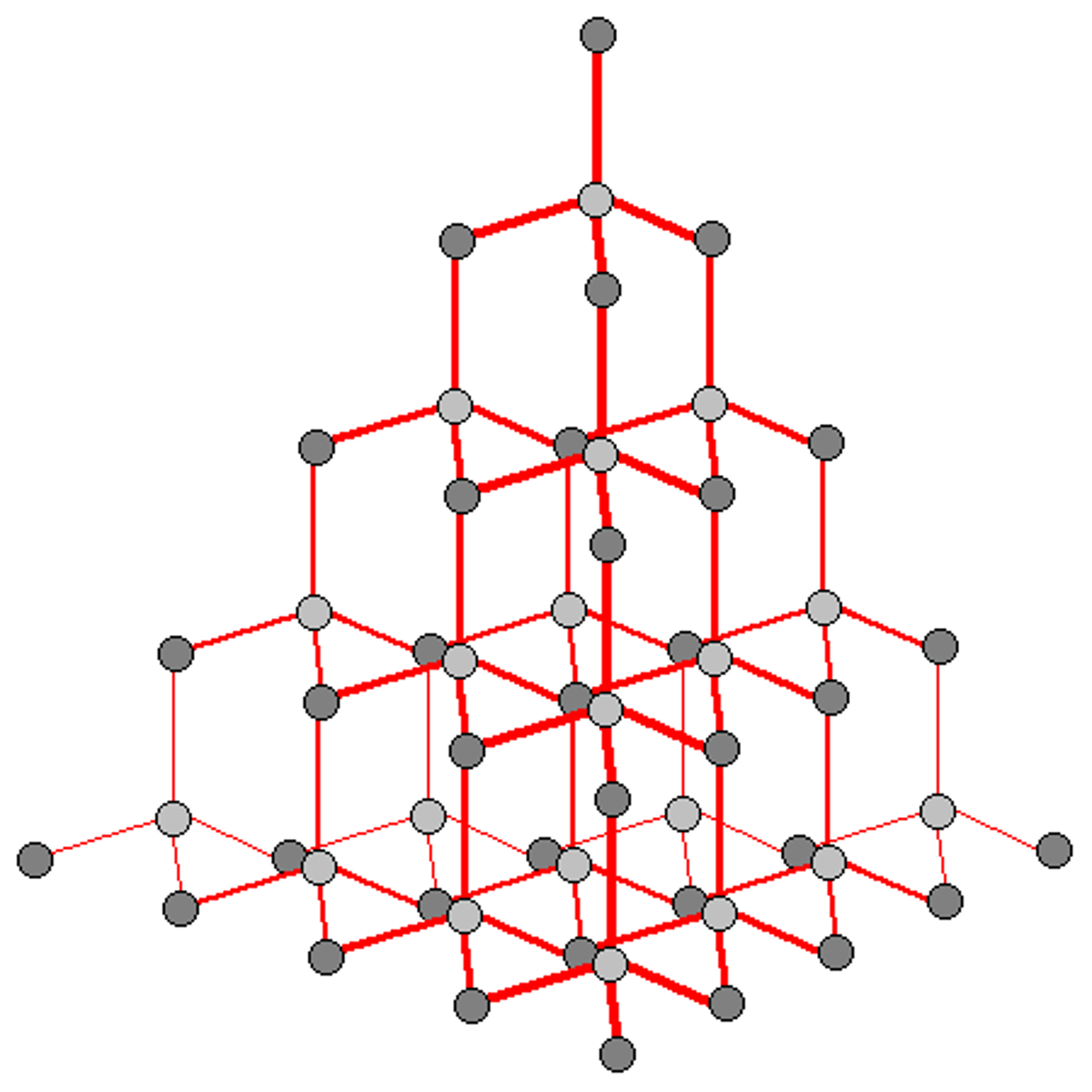
Diamonds requires a great deal of energy to melt because
they have a huge number of covalent bond which needs to be broken, so it has a very high melting/boiling point
Diamond cannot conduct electricity because
all of the outer electrons are in covalent bonds, so there are no free electrons to carry electrical charge
Silicon dioxide contains the elements
silicon and oxygen covalently bonded together
Silicon dioxide have a _______ melting and boiling point
very high
Why does silicon dioxide have a very high melting and boiling point?
a huge number of strong covalent bonds must be broken which takes a lot of energy
what makes something a giant covalent substance?
A giant covalent substance is made up of a huge number of non-metal atoms bonded together by strong covalent bonds in a continuous 3D structure.
give three examples of giant covalent substances:
Diamond, graphite, silicon dioxide (silica)
what are the properties of giant covalent structures?
- high melting and boiling points
- always solid at room temperature
why do giant covalent structures all have high melting/boiling points?
- Giant covalent structures have high melting and boiling points because:
-All the atoms in the structure are joined by strong covalent bonds.
-Covalent bonds require a very large amount of energy to break.
-Since the bonds must be broken (not just weak forces), a lot of heat energy is needed to melt or boil the substance.
what makes something a small molecule?
a molecule is a SMALL molecule ONLY if it contains a FEW atoms. For example, hydrogen only has 2 hydrogen atoms
name all 2 properties of small covalent molecules?
- small covalent molecules have low melting and boiling points
- small covalent molecules do not conduct electricity.
why do small covalent molecules have low melting and boiling points?
- there are weak intermolecular forces between the molecules, and these intermolecular forces do not require a lot of energy to break.
why do small covalent molecules not conduct electricity?
they lack the free electrons to carry an electrical charge.
covalent bonding is when ________ atoms bond together.
non metal
describe a covalent bond
A covalent bond is the electrostatic attraction between a shared pair of electrons and the nuclei of the 2 bonded atoms.
It is formed when 2 atoms share electrons to gain a full outer-shell of electrons.
how many electrons are shared in each covalent bond?
2 electrons
what is a double covalent bond?
A double bond is when there are 2 covalent bonds between the same atoms, meaning there are 4 electrons being shared in total (2 electrons per covalent bond).
describe the covalent bonding of hydrogen
- Two hydrogen atoms overlap their outermost shells.
- Each hydrogen atom shares its one and only electron with the other.
- so each hydrogen atom is then in contact with 2 electrons - the maximum number that the first shell can hold!
-so now,the molecule is more stable than the individual hydrogen atoms.
describe the covalent bonding of H2O.
- Each hydrogen atom has one electron, and the oxygen atom has six electrons in its outer shell
To achieve stability:
- Each hydrogen atom shares its one electron with the oxygen atom.
- In turn, the oxygen atom shares one of its electrons with each hydrogen atom.
- This sharing forms a double covalent bond.
As a result:
Each hydrogen atom has a full outer shell (two electrons).
The oxygen atom has a full outer shell (eight electrons).
describe the covalent bonding of nitrogen (N2).
Here's how it happens:
- Each nitrogen atom has 5 electrons in its outer shell
- To achieve a full outer shell, each nitrogen atom shares 3 electrons with the other.
- This sharing of three pairs of electrons forms a triple covalent bond between the two nitrogen atoms (written as N≡N).
Each hydrogen atom has a full outer shell (two electrons).
The oxygen atom has a full outer shell (eight electrons).
EXAM QUESTION: explain, in terms of electrons and chemical bonds, what happens when potassium reacts with fluorine to form potassium fluoride.
- potassium is in group 1. It has one outer shell electron.
- Fluorine is in group 7. It has seven outer shell electrons.
- In order for both elements to achieve a full outer shell, potassium donates its one outer shell electron to fluorine.
- this means that the potassium atoms become positively charged potassium ions and the fluorine atoms become negatively charged fluorine ions.
name 2 properties of ionic compounds.
- ionic compounds have very high melting and boiling points. this is because the strong electrostatic forces of attraction require a great deal of heat energy to break.
- they cannot conduct electricity when they are solids. this is because when in a solid, the ions (NOT ELECTRONS) are locked in place by the strong electrostatic forces of attraction.
when does ionic bonding take place?
when a metal and non metal react
ionic bonding: lithium and fluoride. go.
lithium is 2,1. fluoride is 2,7.
so lithium gives its one electron to fluoride so fluoride can have a full outer shell. so when that happens, lithium develops one positive charge and fluoride develops one negative charge.
the particle model DOES NOT take into account... (four things)
- the forces between particles
- the size of particles
- the space between particles
- the fact that melting/boiling points vary between substances
the particles model assumes... (two things)
- atoms are solid spheres
- there are no forces between them
SOLIDS? (four points) (PAN M)
- arranged in a fixed position
- very close together
- stay fixed to their neighbour
- can only vibrate
LIQUIDS? (four points) (PAN M)
- randomly arranged
- are close together
- always change neighbour
- can move around freely
GASES? (three points) (PAN M)
- randomly arranged
- lots of space between each other
- always change neighbour
- move around freely and very quickly
how does freezing/condensing work? (both work the same way)
cooling -> decreased energy -> particles move less -> particles stop moving around and just vibrate, forming a fixed shape attraction between particles form
how does melting/boiling work? (both work the same way)
heated -> increased energy -> particles vibrate more -> individual particles start to break free of the structure, forces are overcome
what is the reactivity of group one metals? (does it increase/decrease down the group?)
The reactivity increases down the group
-the atoms get bigger
-so the outer electrons are further from the protons
-so the force beetween them is weaker
-so the electron is lost more easily
What are group one metals stored in?
Oil
Alkali metal + water = ???
metal hydroxide + hydrogen
Alkali metal + oxygen = ???
alkali metal oxide
Alkali metal + halogen (group 7) = ???
Alkali metal halide
what is the reactivity of group 7 halogens? (does it increase/decrease down the group?)
decreases as you go down the group
what is halogen displacement?
A reaction in which a more reactive halogen takes the place of a less reactive halogen from its compound.
EXAMPLE: chlorine + potassium bromide
potassium chloride + bromide
Where are the transition metals located?
In that large block between group 2 and 3
name all 4 physical properties' of transition metals (CSDM/B)
- good conductor of electricity and thermal energy
- hard and strong
- high density
- high melting points
what are chemical properties of transition metals?
- MUCH less reactive than the metals in group 1
what is the reactivity of noble gases (group 0)
Very unreactive
Because they all have complete outer shells, so they don't want to lose or gain electrons. (bros js a chill guy)
John Newlands (what was his thing called) (2 things he did and 2 howevers)
THE LAW OF OCTAVES
-ordered the elements in INCREASING ORDER OF ATOMIC MASS
- noticed every 8th element had similar properties
HOWEVER...
- he assumed that all the elements were found
- this rule only worked until calcium
Dmitri Mendeleev
-ordered the elements based on atomic weight
- if he needed to, he would switch the order of specific elements so they fitted the patterns
- he left gaps in his periodic table for elements that hadn't been discovered yet
- he even predicted the properties of the undiscovered elements (YES HE WAS THAT CONFIDENT)
what is the MAXIMUM amount of electrons that can be on the first shell?
2
what is the MAXIMUM amount of electrons that can be on the rest of the shells?
8
how would you find the number of shells/orbitals in a element using a periodic table?
period (row) number
how would you find the number of electrons in the last shell of an element using a periodic table?
group (column) number
how would you find the number of electrons in TOTAL of an element using a periodic table?
atomic (bottom) number
why do elements in the same group has similar chemical properties?
when atoms collide/react, the outer electrons are the ones that acc interact. that's why elements in the same group has similar properties, because they have the same number of electrons in their outer shells.