VSEPR Theory
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Linear bond angle:
180°
Trigonal bond angle:
120°
Tetrahedral bond angle:
109.5°
Trigonal bipyramidal bond angles (equatorial and axial):
Equatorial-Equatorial: 120°
Axial-Equatorial: 90°
Axial-Axial: 180°
Octahedral bond angle:
90°
Bent bond angle:
<120° (atom-atom)
Trigonal pyramidal bond angle:
<109.5° (atom-atom)
Seesaw/Sawhorse bond angles (equatorial and axial):
Equatorial-Equatorial: <120° (atom-atom)
Axial-Equatorial: <90° (atom-atom)
Axial-Axial: <180° (atom-atom)
T-shaped bond angles (equatorial and axial):
Equatorial-Equatorial: <120° (atom-electron group)
Axial-Equatorial: <90° (atom-atom)
Axial-Axial: <180° (atom-atom)
Square pyramidal bond angle:
<90° (atom-atom)
Square planar bond angle:
90° (atom-atom)
Name of VSEPR shape with 2 bonds:
Linear
Name of VSEPR shape with 3 bonds:
Trigonal Planar
Name of VSEPR shape with 4 bonds:
Tetrahedral
Name of VSEPR shape with 5 bonds:
Trigonal Bipyramidal
Name of VSEPR shape with 6 bonds:
Octahedral
Valence Bond Theory's orbital hybridization associated with the Linear VSEPR shape:
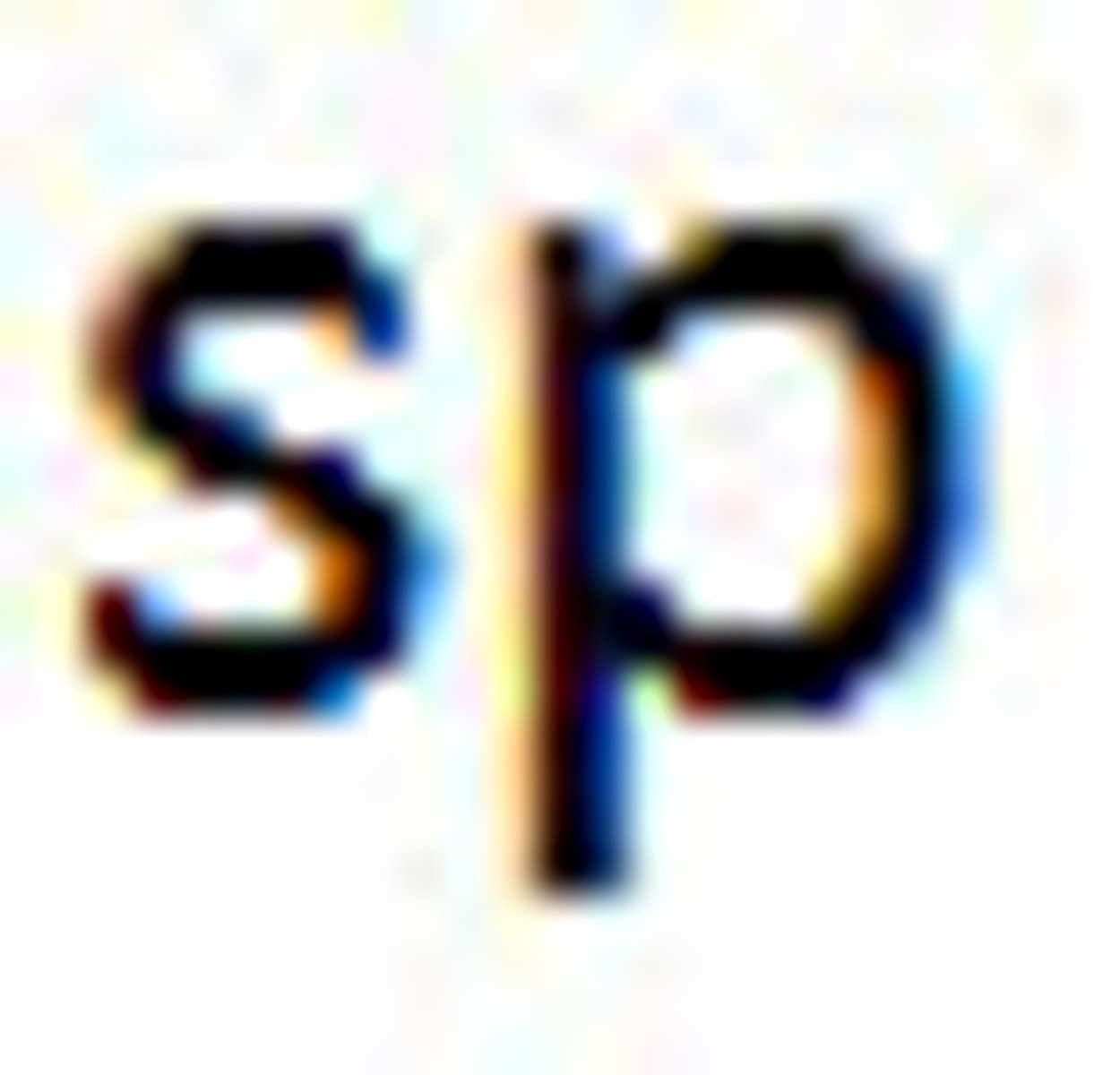
Valence Bond Theory's orbital hybridization associated with the Trigonal Planar VSEPR shape:
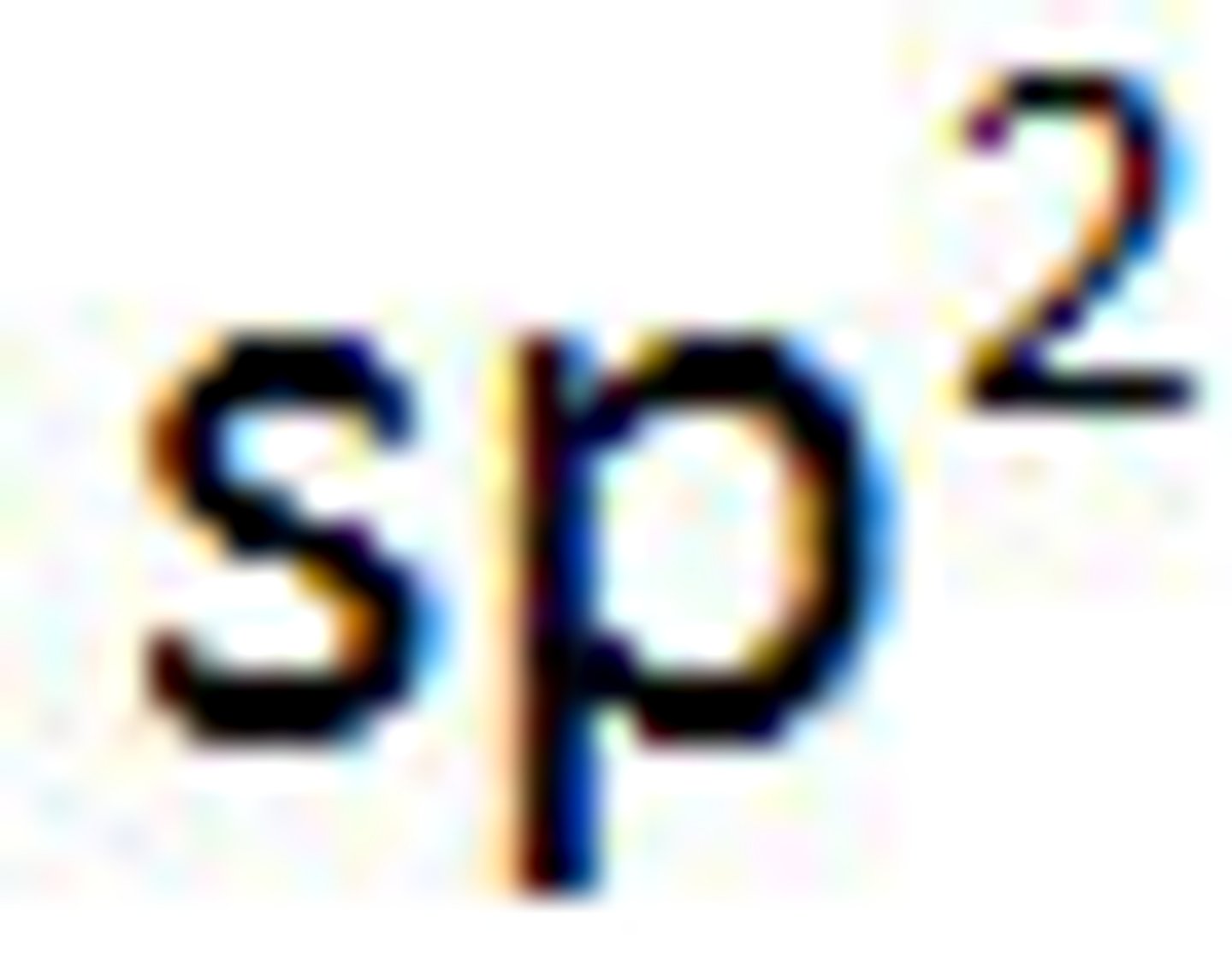
Valence Bond Theory's orbital hybridization associated with the Tetrahedral VSEPR shape:
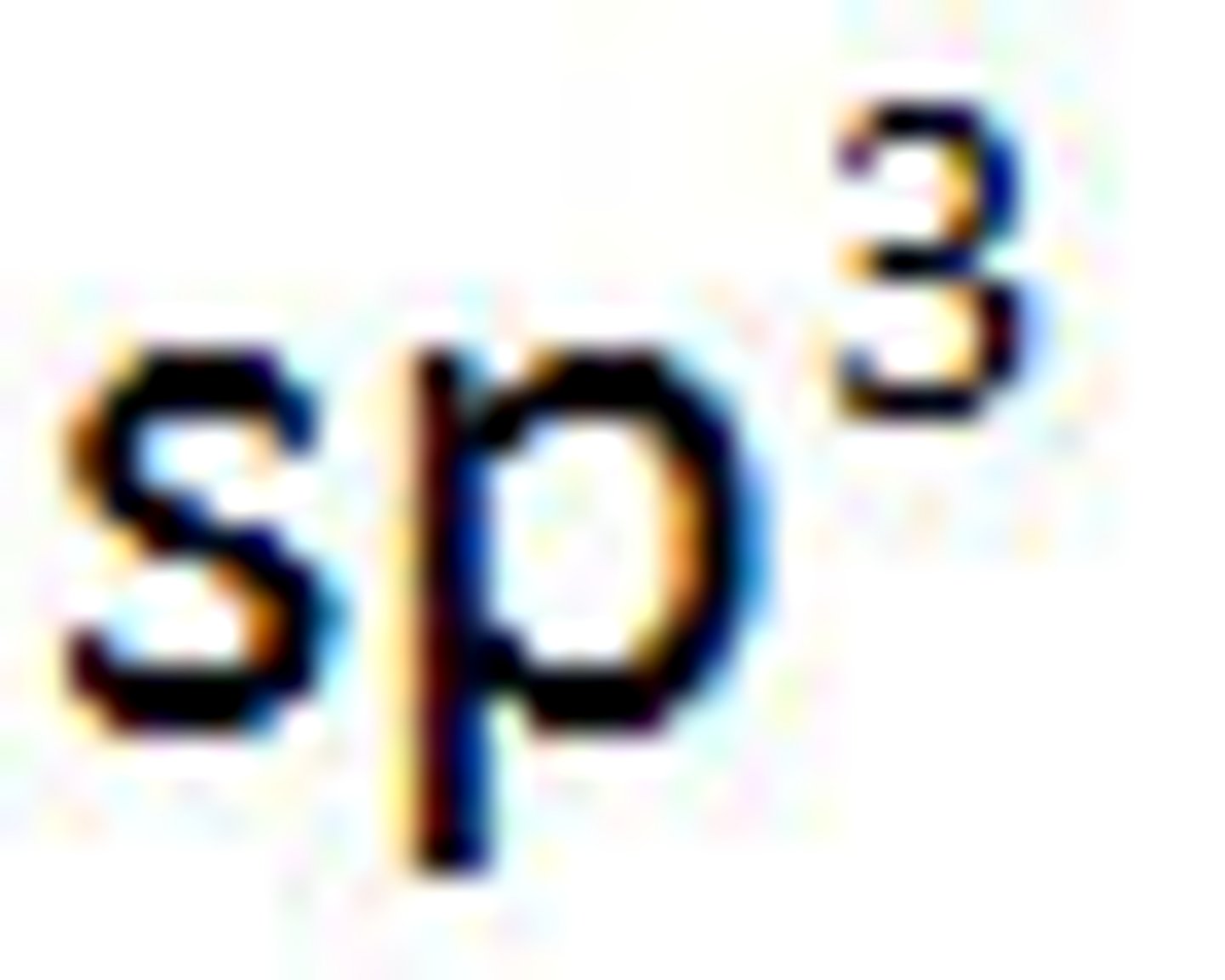
Valence Bond Theory's orbital hybridization associated with the Trigonal Bipyramidal VSEPR shape:
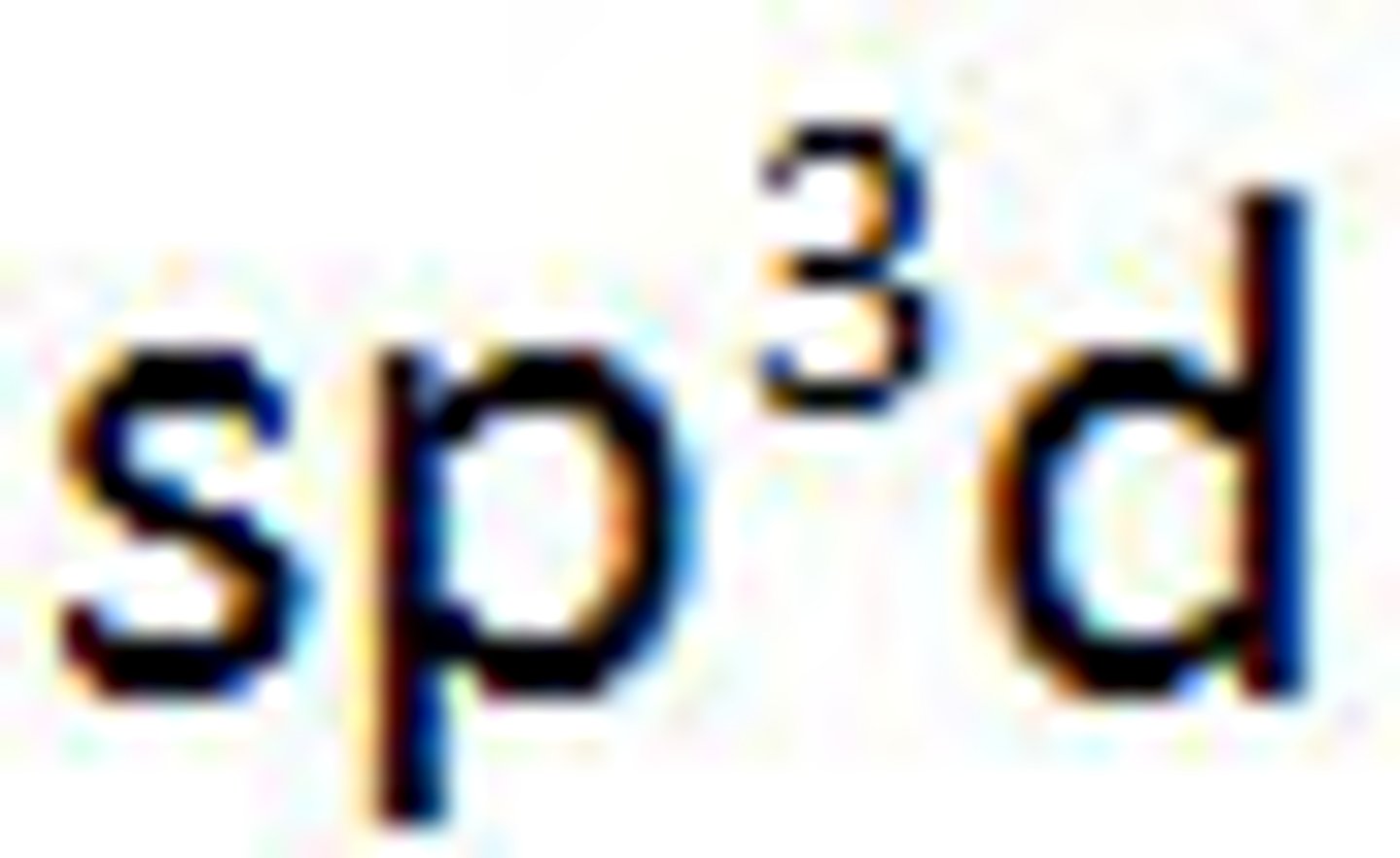
Valence Bond Theory's orbital hybridization associated with the Octahedral VSEPR shape:
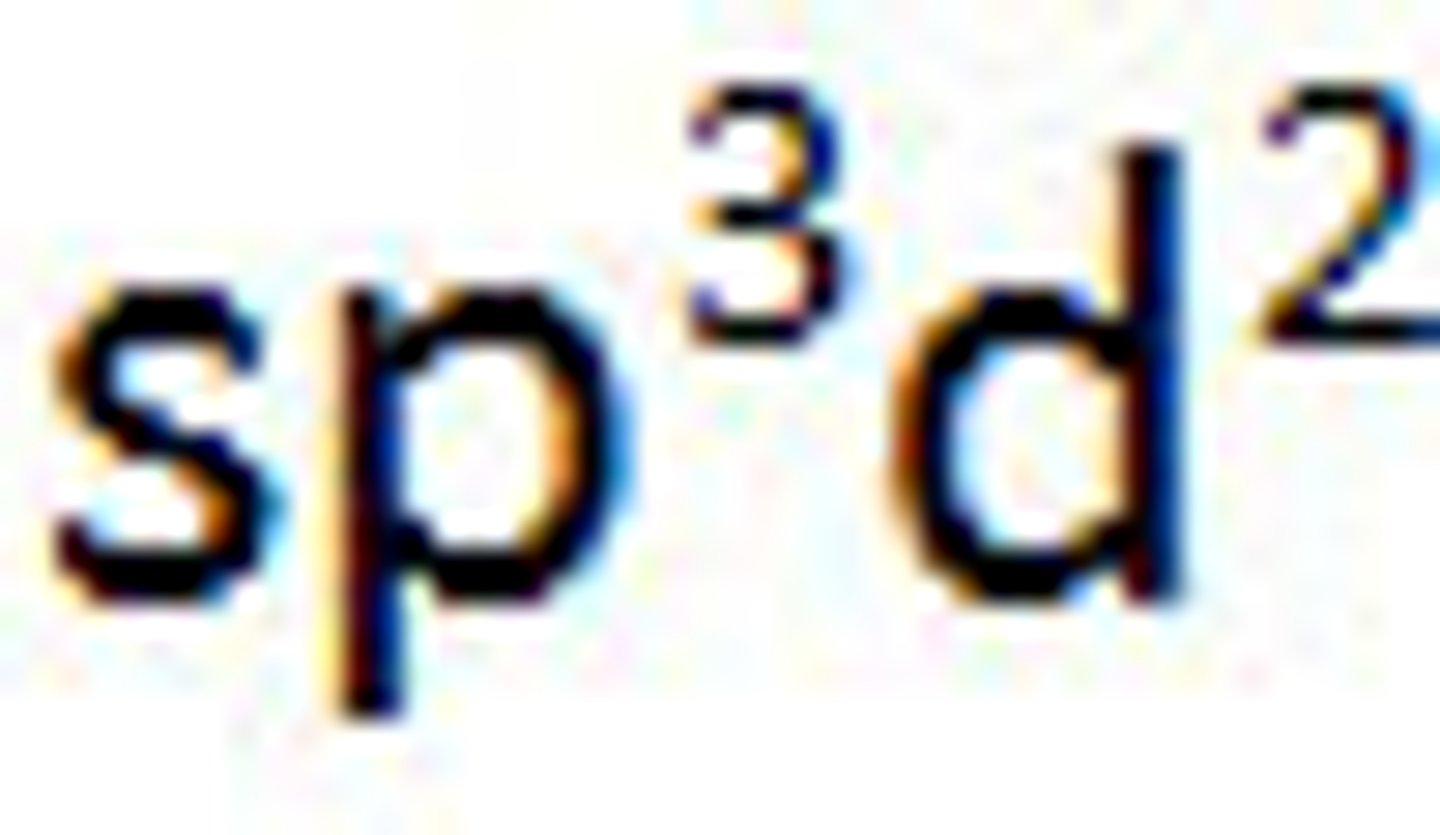
Name of VSEPR shape with 2 bonds and 1 lone pair:
Bent
Name of VSEPR shape with 3 bonds and 1 lone pair:
Trigonal Pyramidal
Name of VSEPR shape with 2 bonds and 2 lone pairs:
Bent
Name of VSEPR shape with 4 bonds and 1 lone pair:
Seesaw/Sawhorse
Name of VSEPR shape with 3 bonds and 2 lone pairs:
T-Shaped
Name of VSEPR shape with 2 bonds and 3 lone pairs:
Linear
Name of VSEPR shape with 5 bonds and 1 lone pair:
Square Pyramidal
Name of VSEPR shape with 4 bonds and 2 lone pairs:
Square Planar
Name of VSEPR shape with 3 bonds and 3 lone pairs:
T-Shaped
Name of VSEPR shape with 2 bonds and 4 lone pairs:
Linear
Diagram of VSEPR shape with 2 bonds and 0 lone pairs:

Diagram of VSEPR shape with 3 bonds and 0 lone pairs:
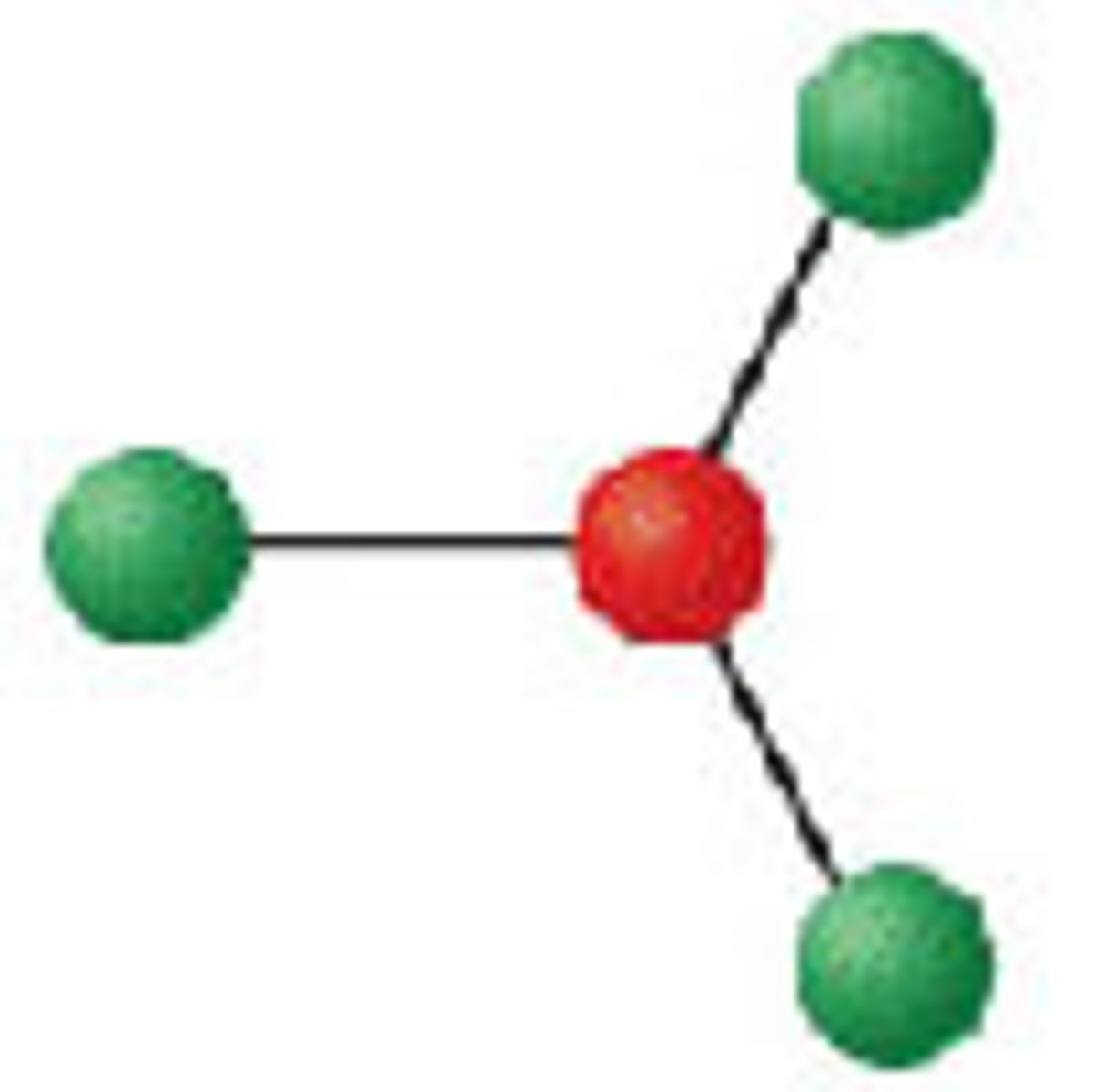
Diagram of VSEPR shape with 2 bonds and 1 lone pair:
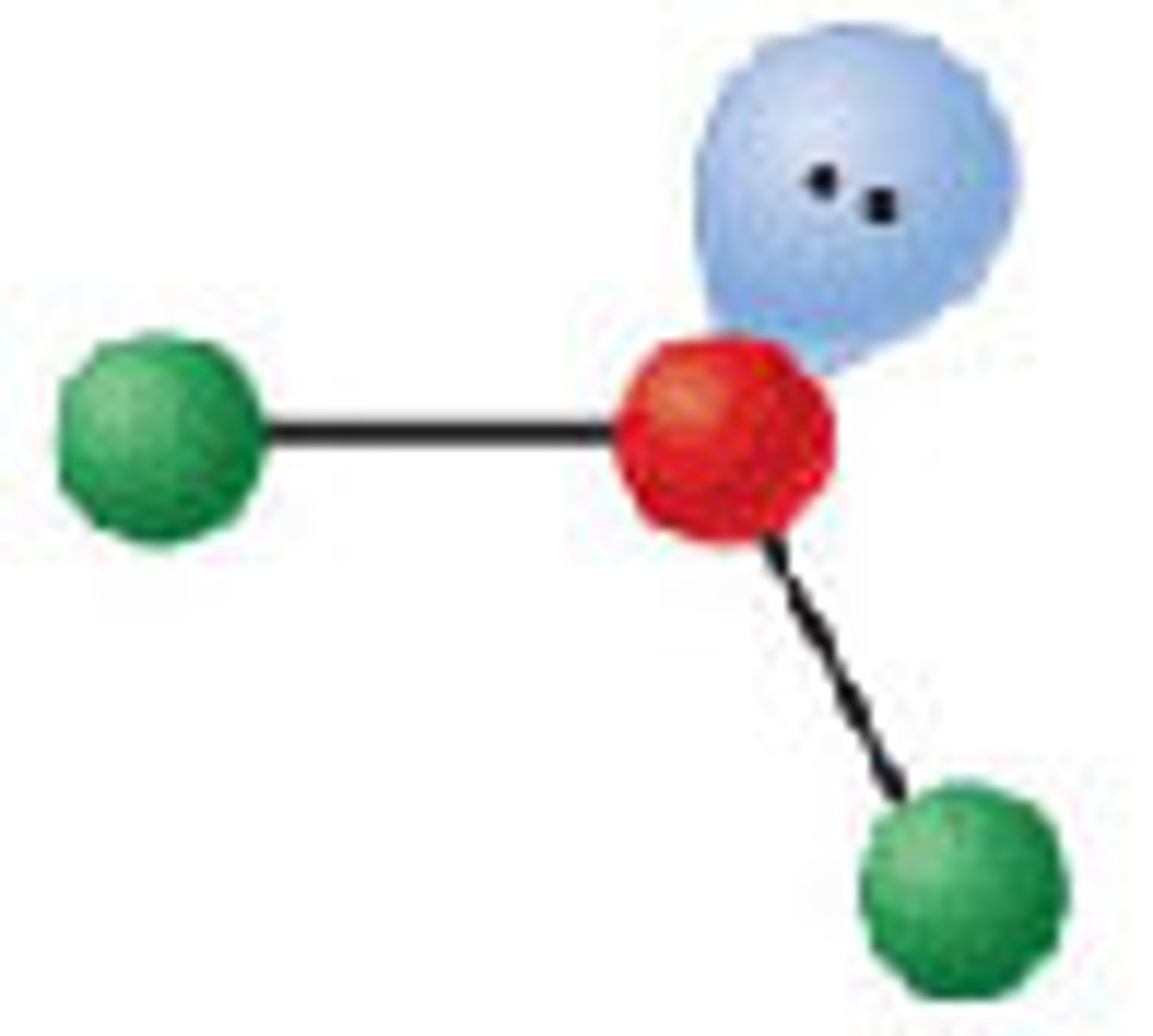
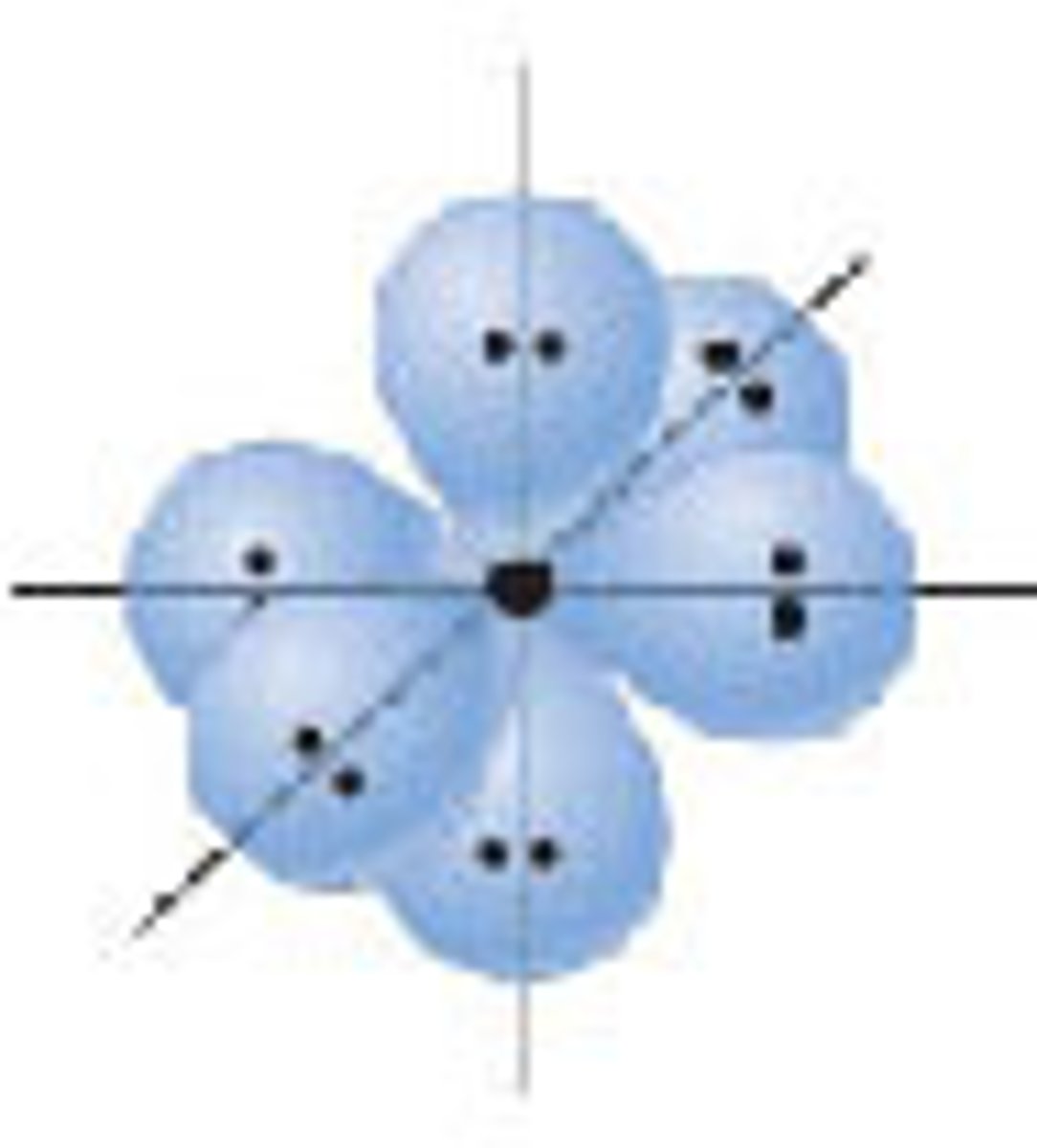
Diagram of VSEPR shape with 4 bonds and 0 lone pairs:
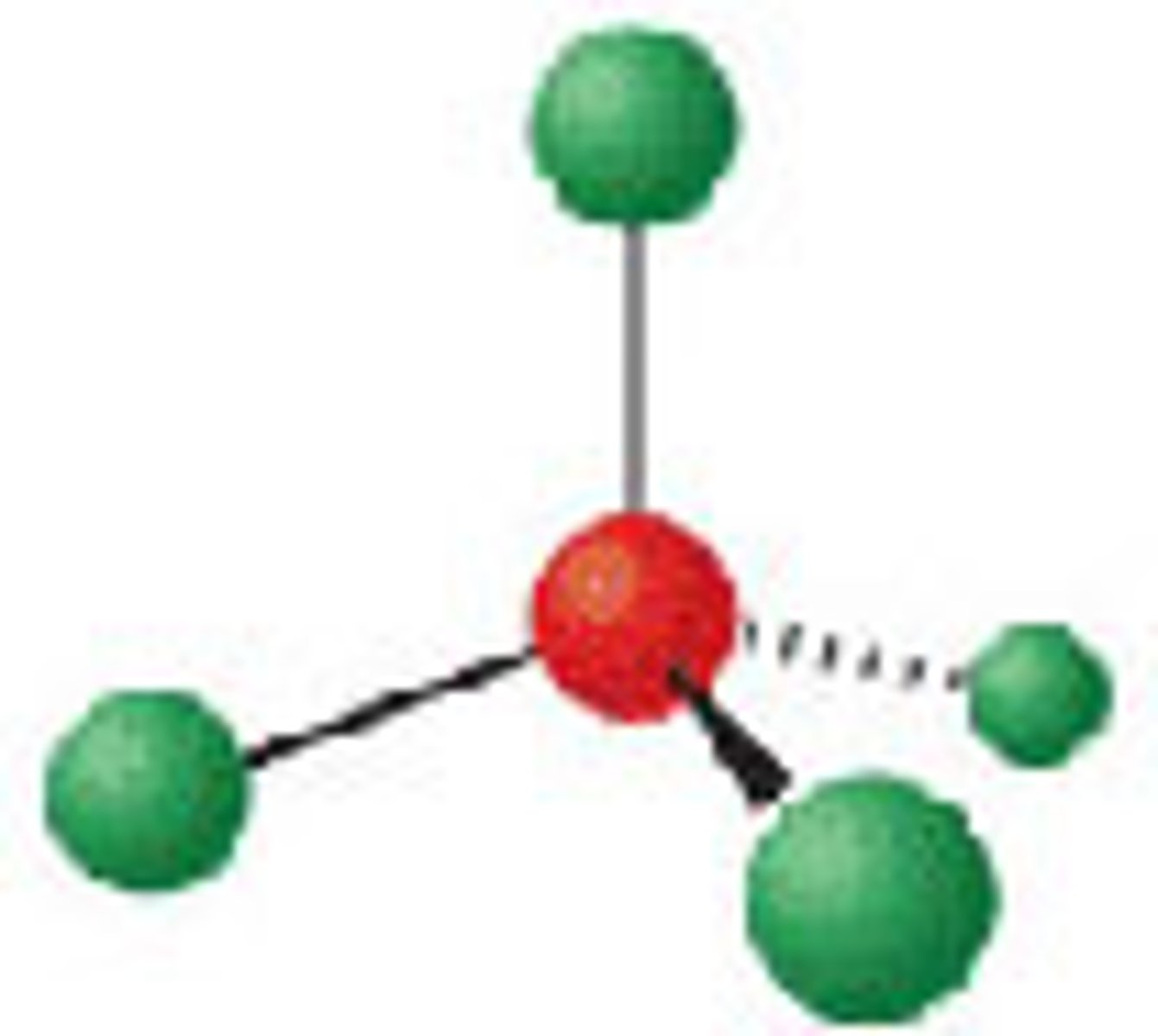
Diagram of VSEPR shape with 3 bonds and 1 lone pair:
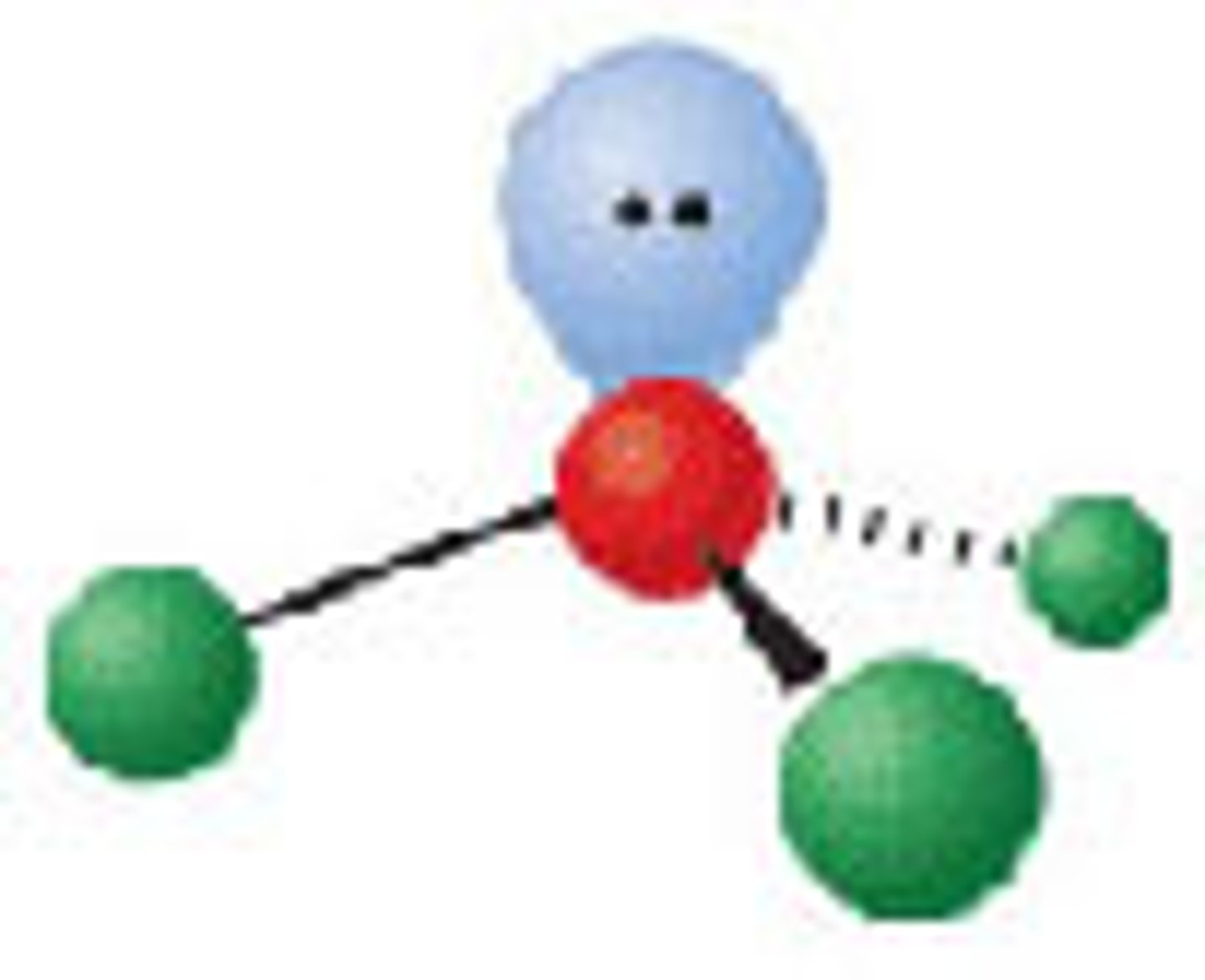
Diagram of VSEPR shape with 2 bonds and 2 lone pairs:

Diagram of VSEPR shape with 5 bonds and 0 lone pairs:
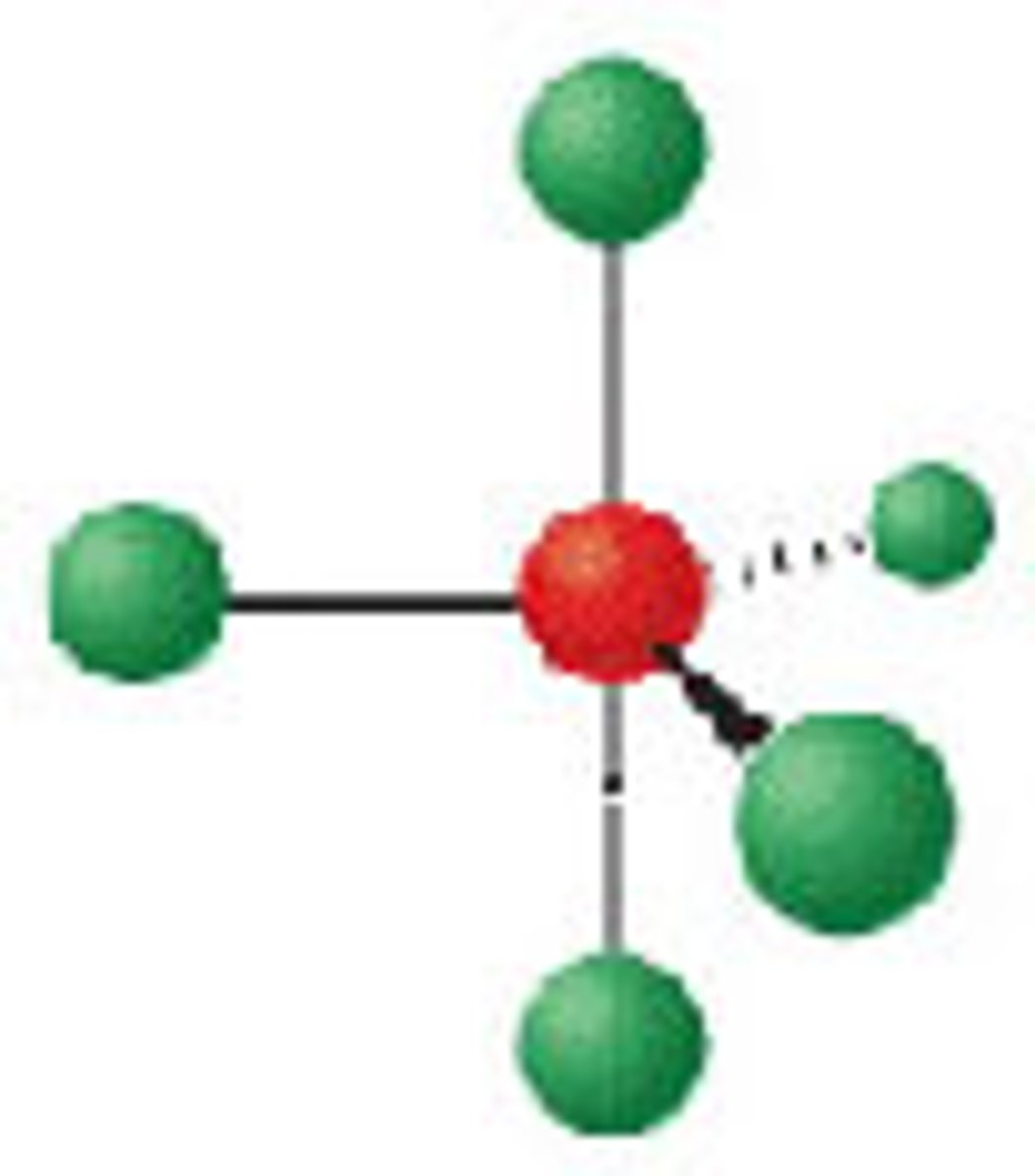
Diagram of VSEPR shape with 4 bonds and 1 lone pair:
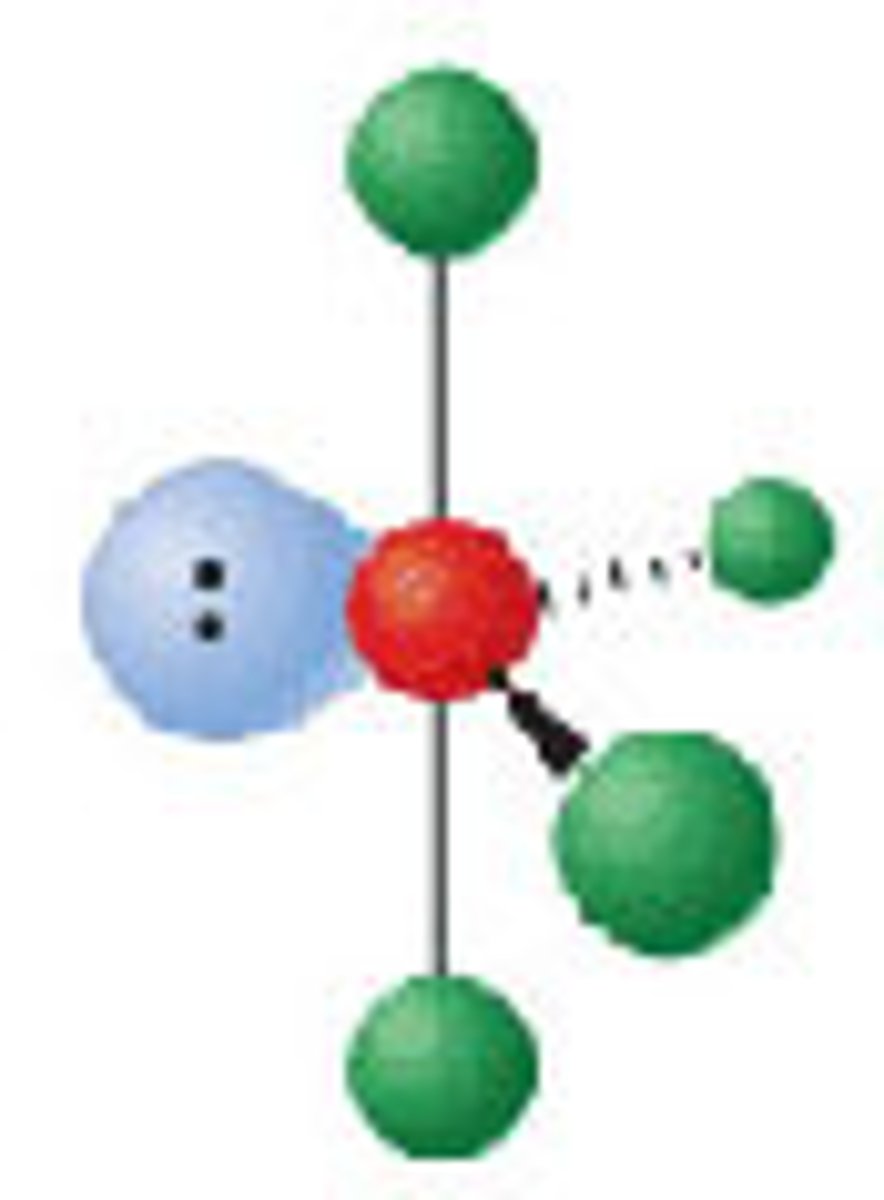
Diagram of VSEPR shape with 3 bonds and 2 lone pairs:
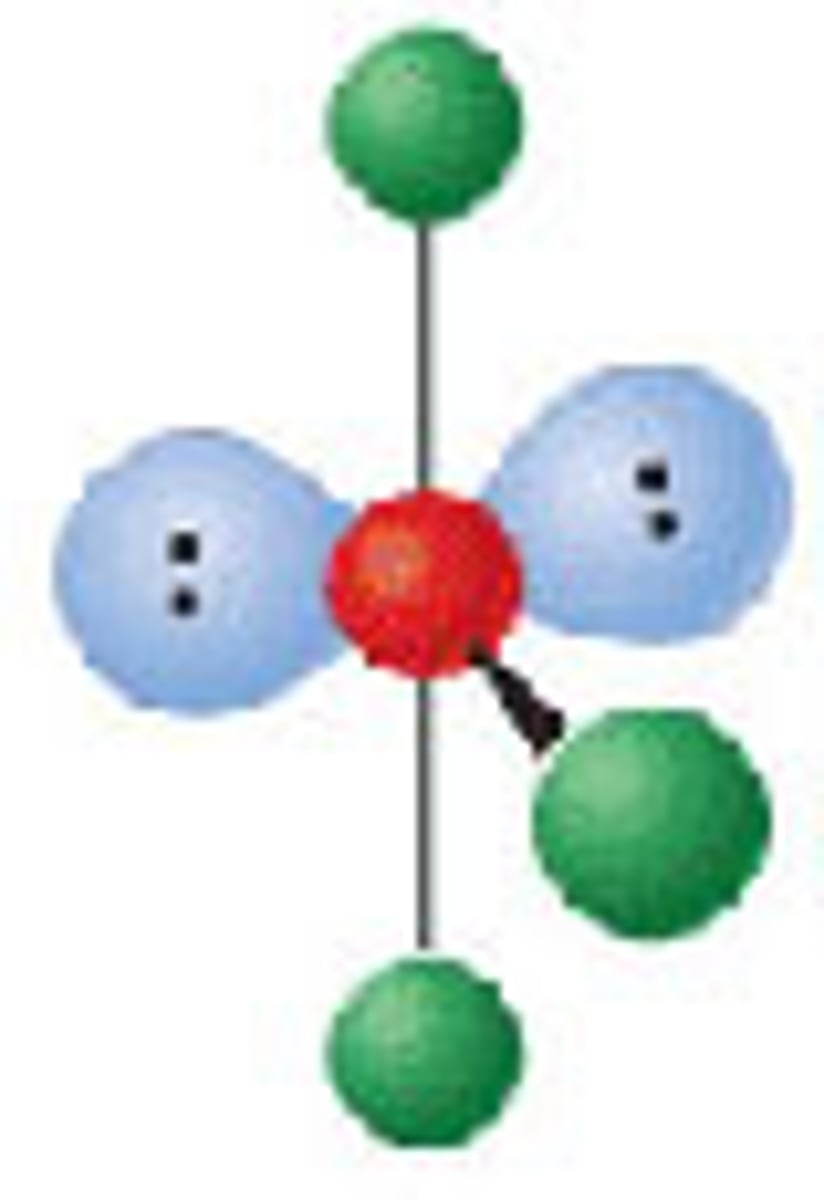
Diagram of VSEPR shape with 2 bonds and 3 lone pairs:

Diagram of VSEPR shape with 6 bonds and 0 lone pairs:
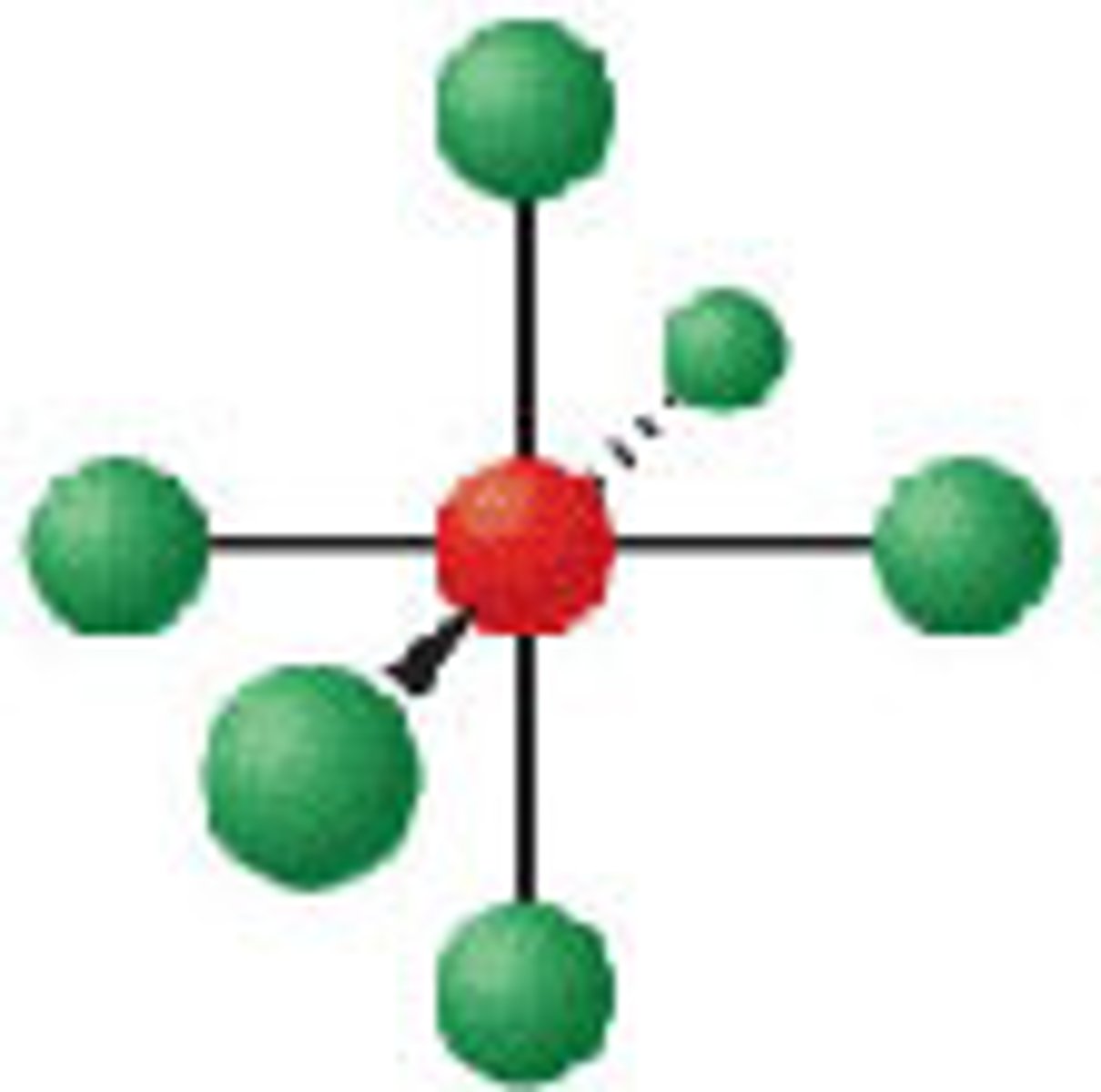
Diagram of VSEPR shape with 5 bonds and 1 lone pair:
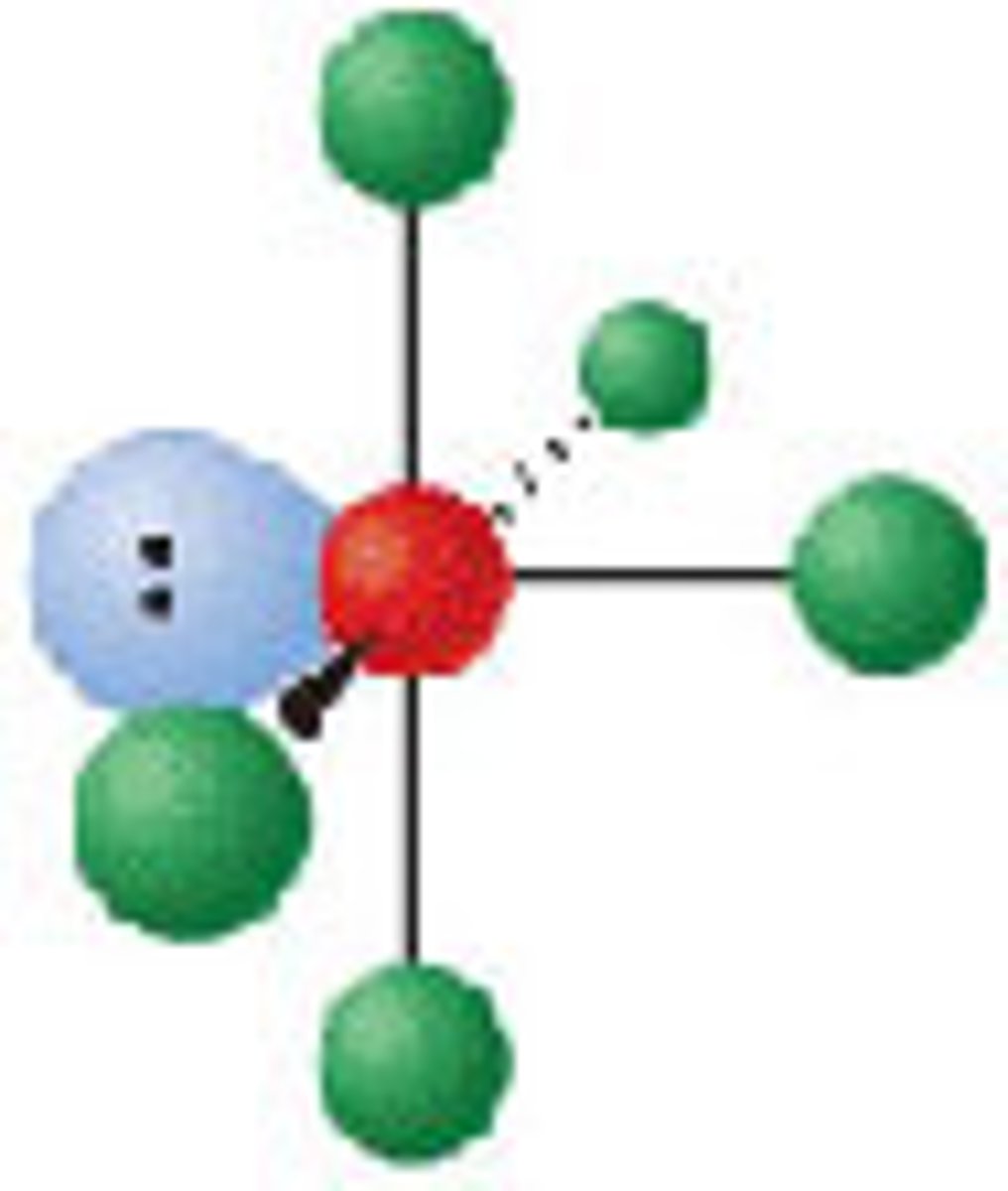
Diagram of VSEPR shape with 4 bonds and 2 lone pairs:
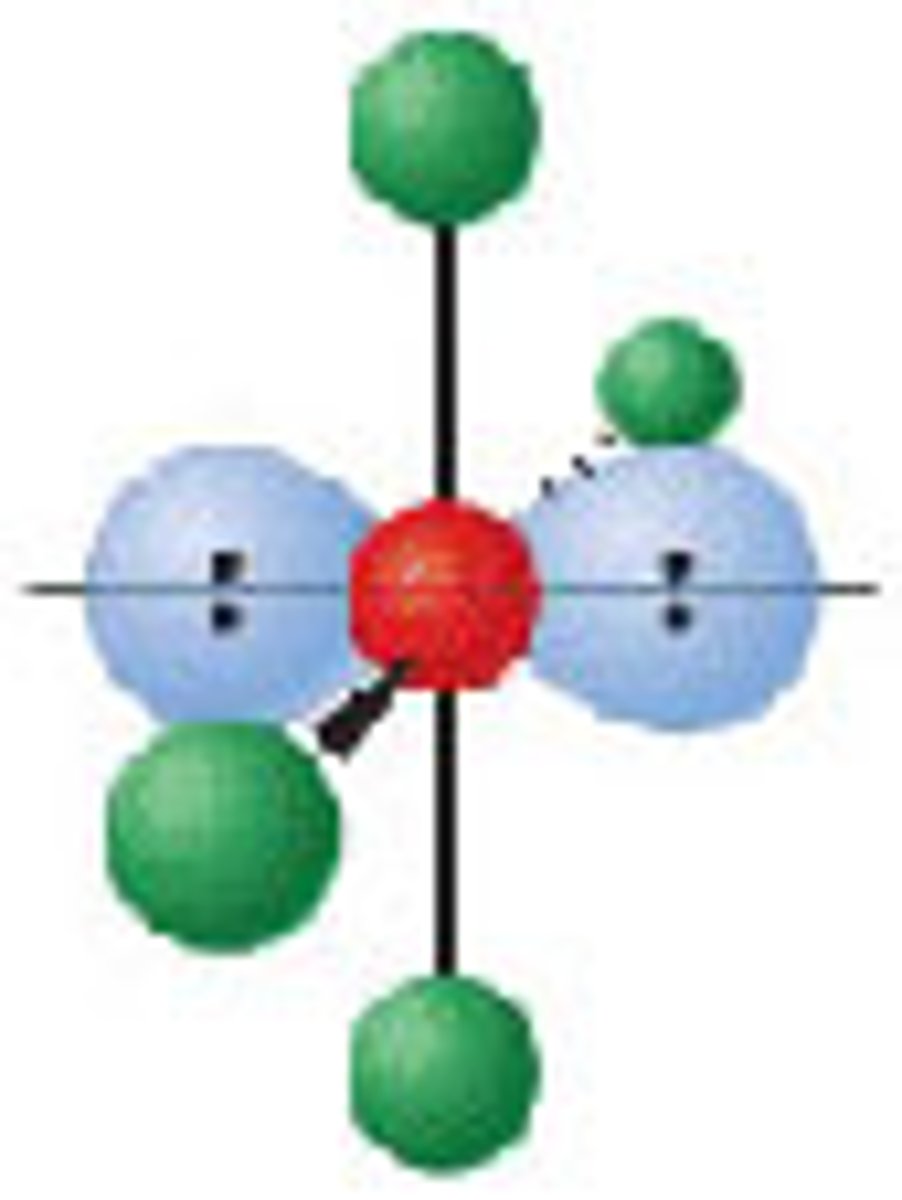
Diagram of VSEPR shape with 3 bonds and 3 lone pairs:
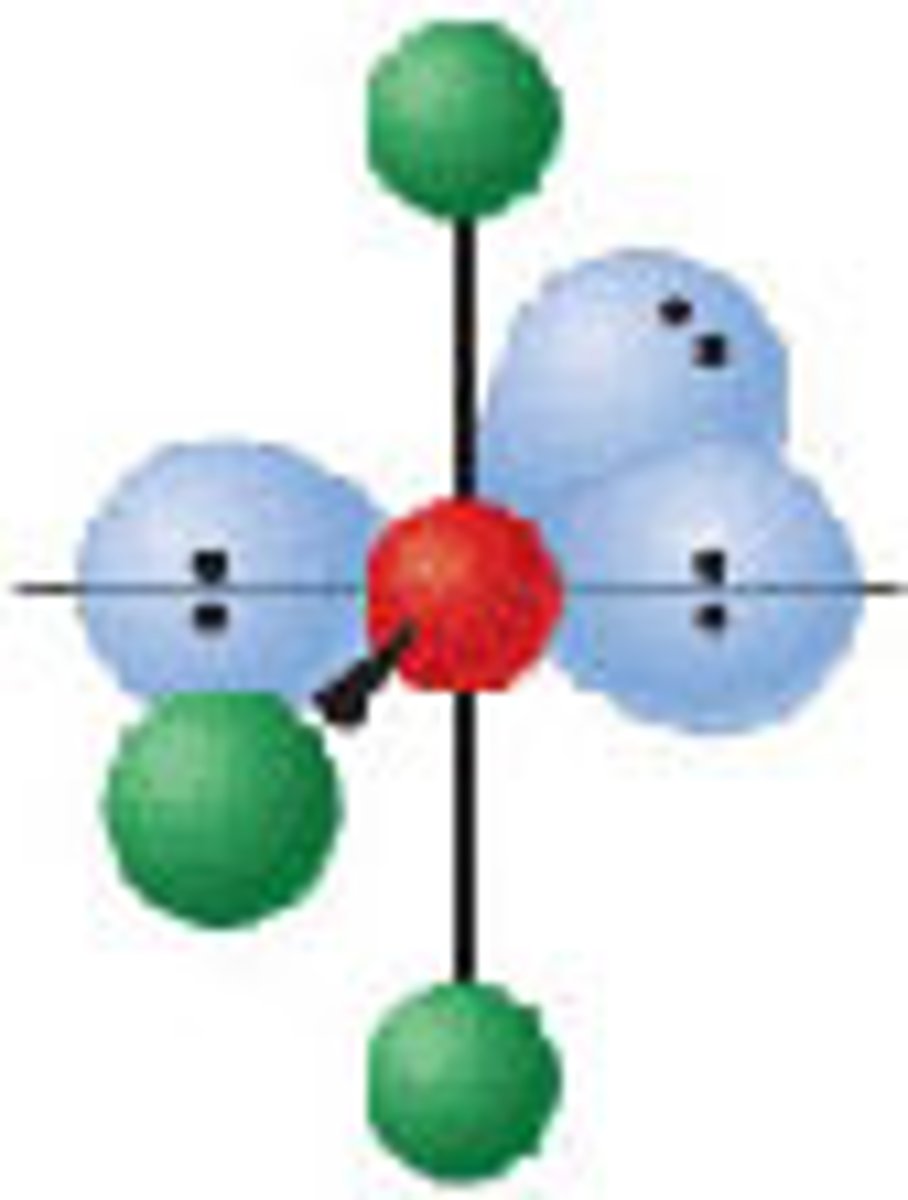
Diagram of VSEPR shape with 2 bonds and 4 lone pairs:
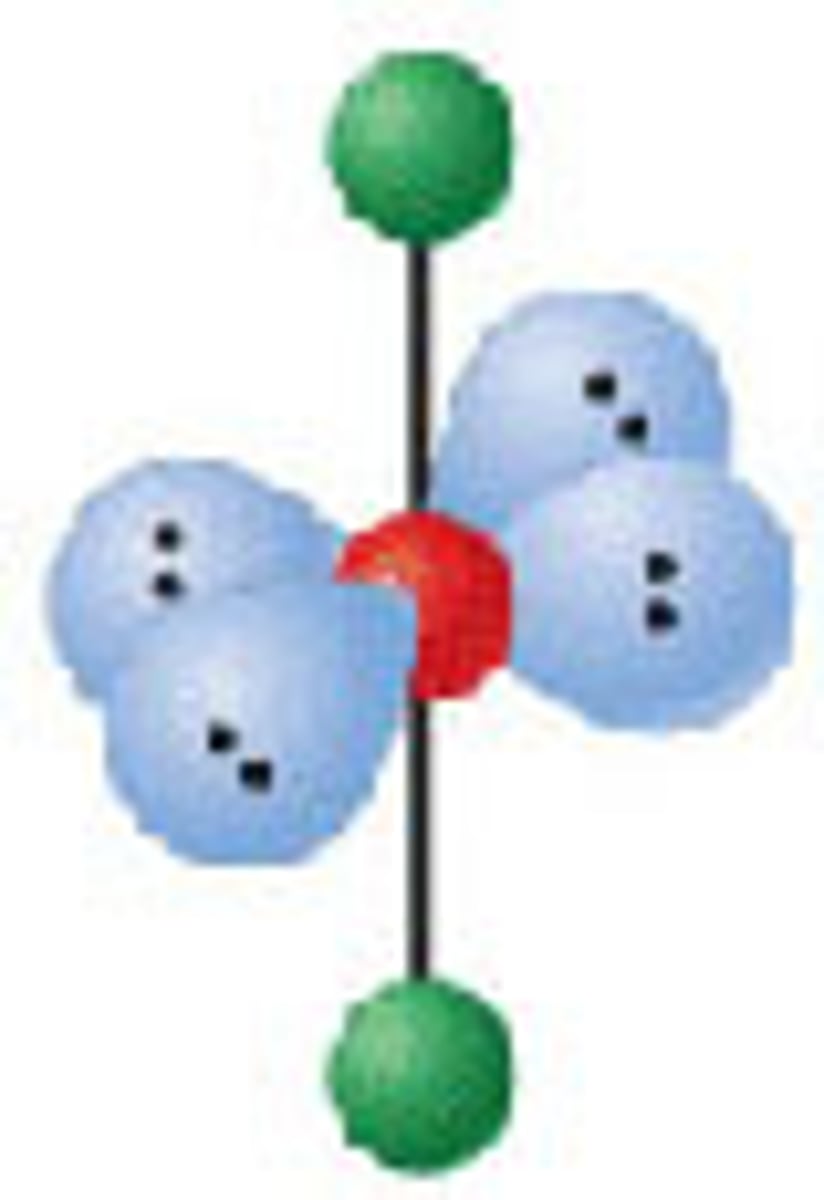
Diagram of Valence Theory hybridization orbital sp:
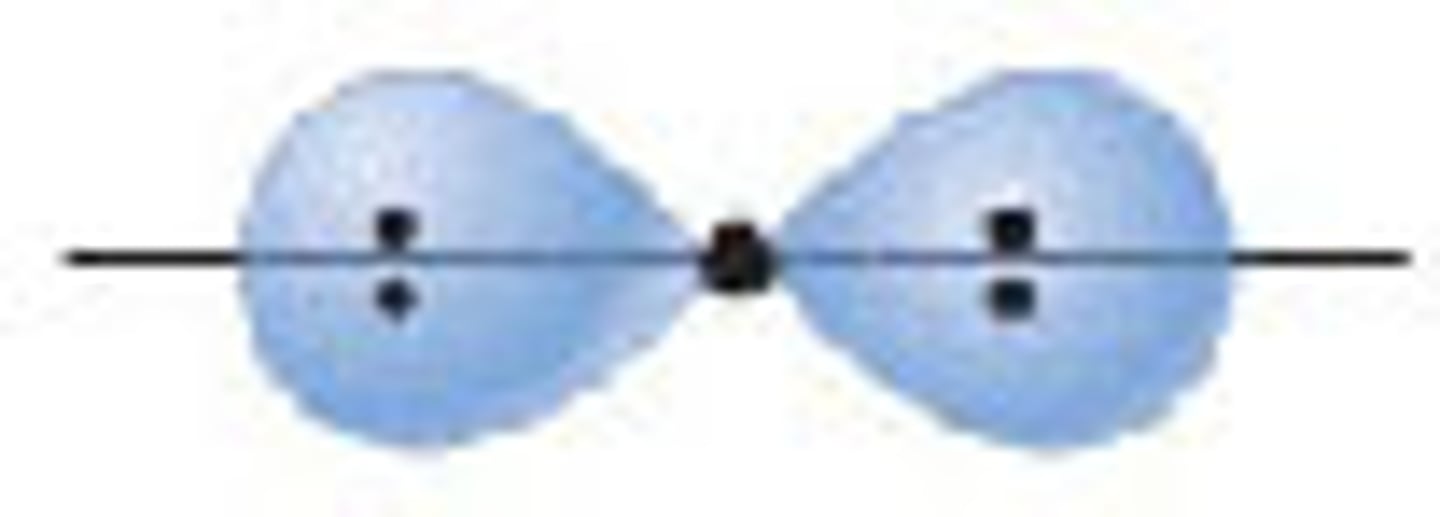
Diagram of Valence Theory hybridization orbital sp2:
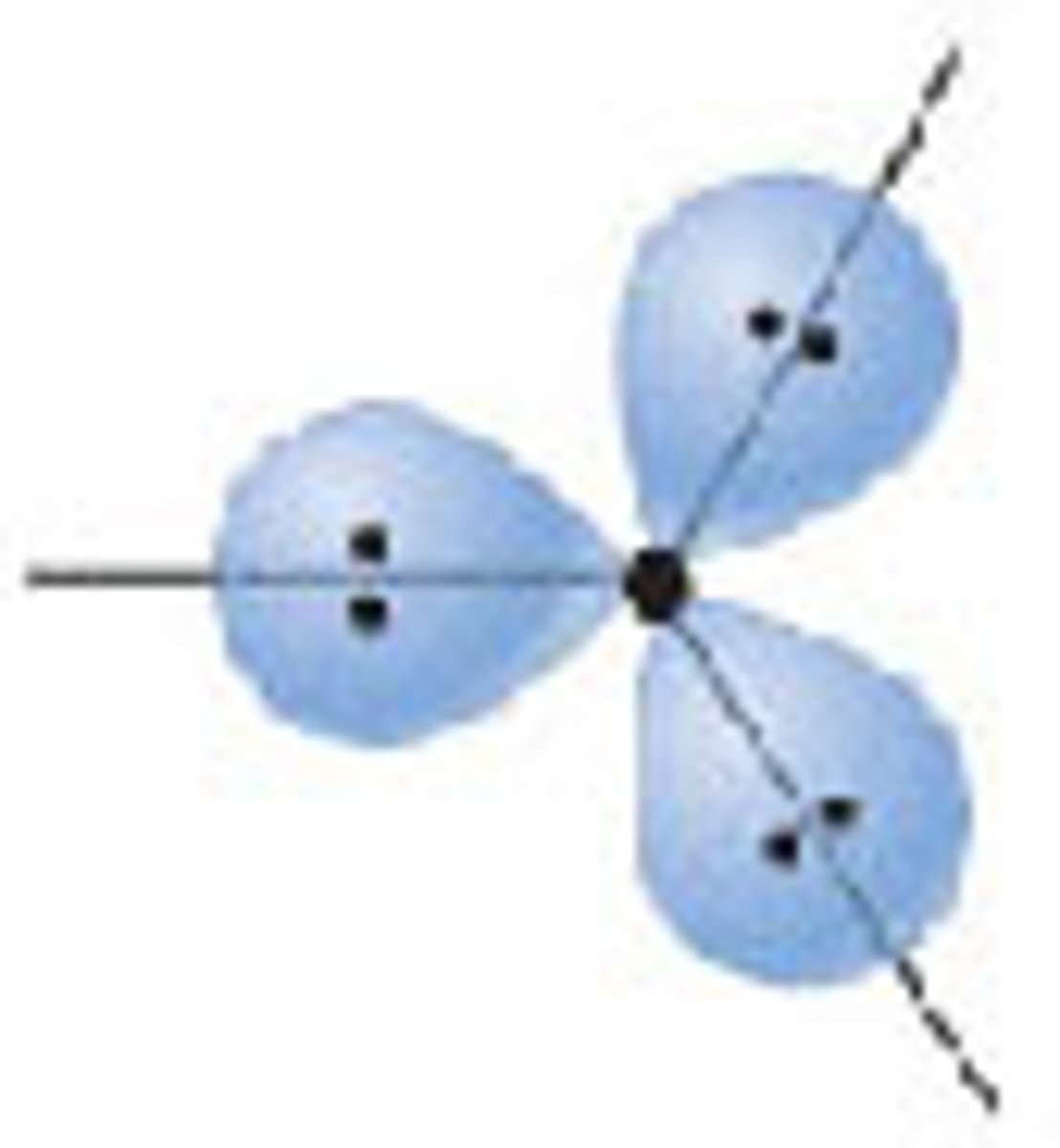
Diagram of Valence Theory hybridization orbital sp3:
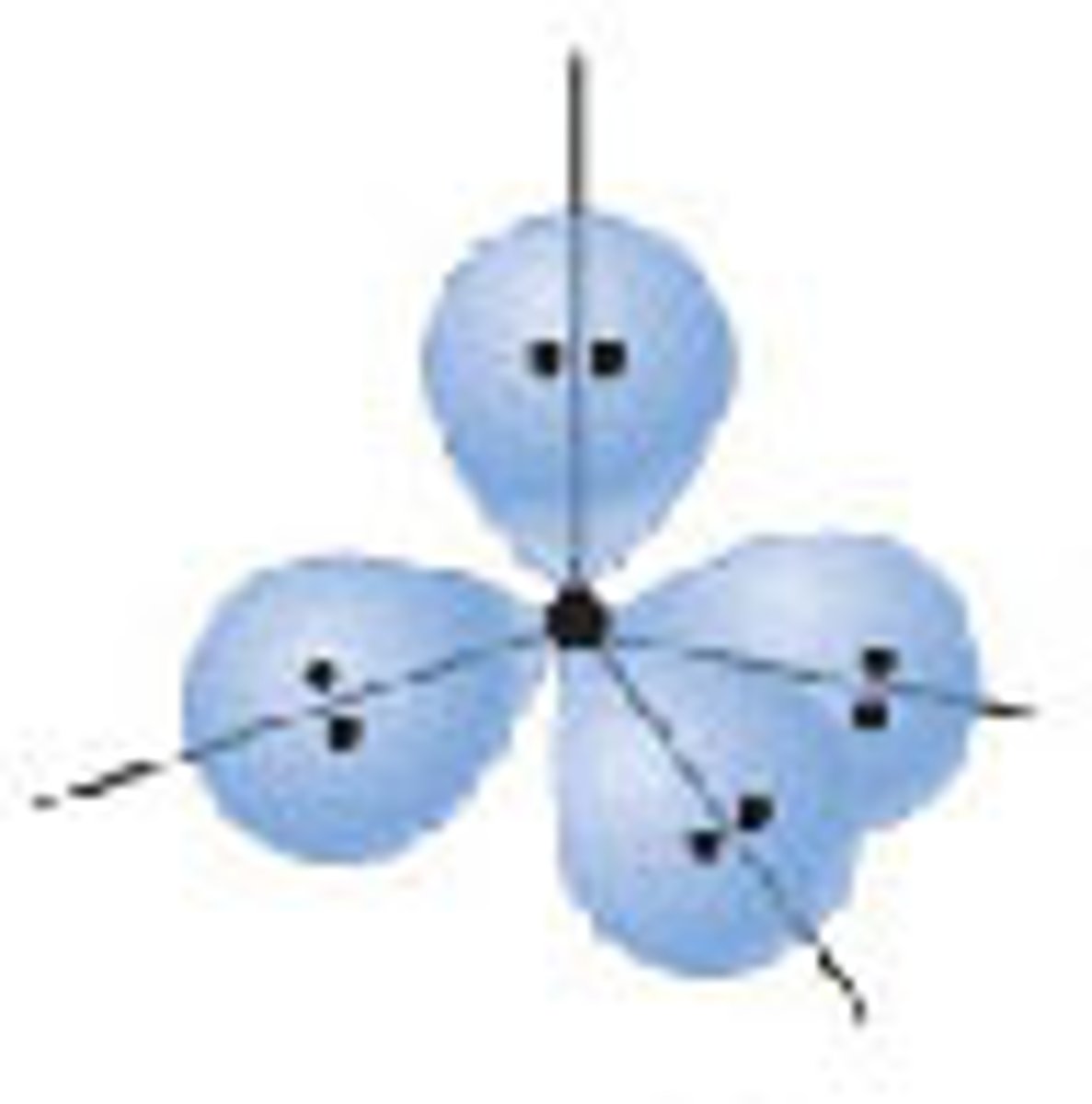
Diagram of Valence Theory hybridization orbital sp3d:
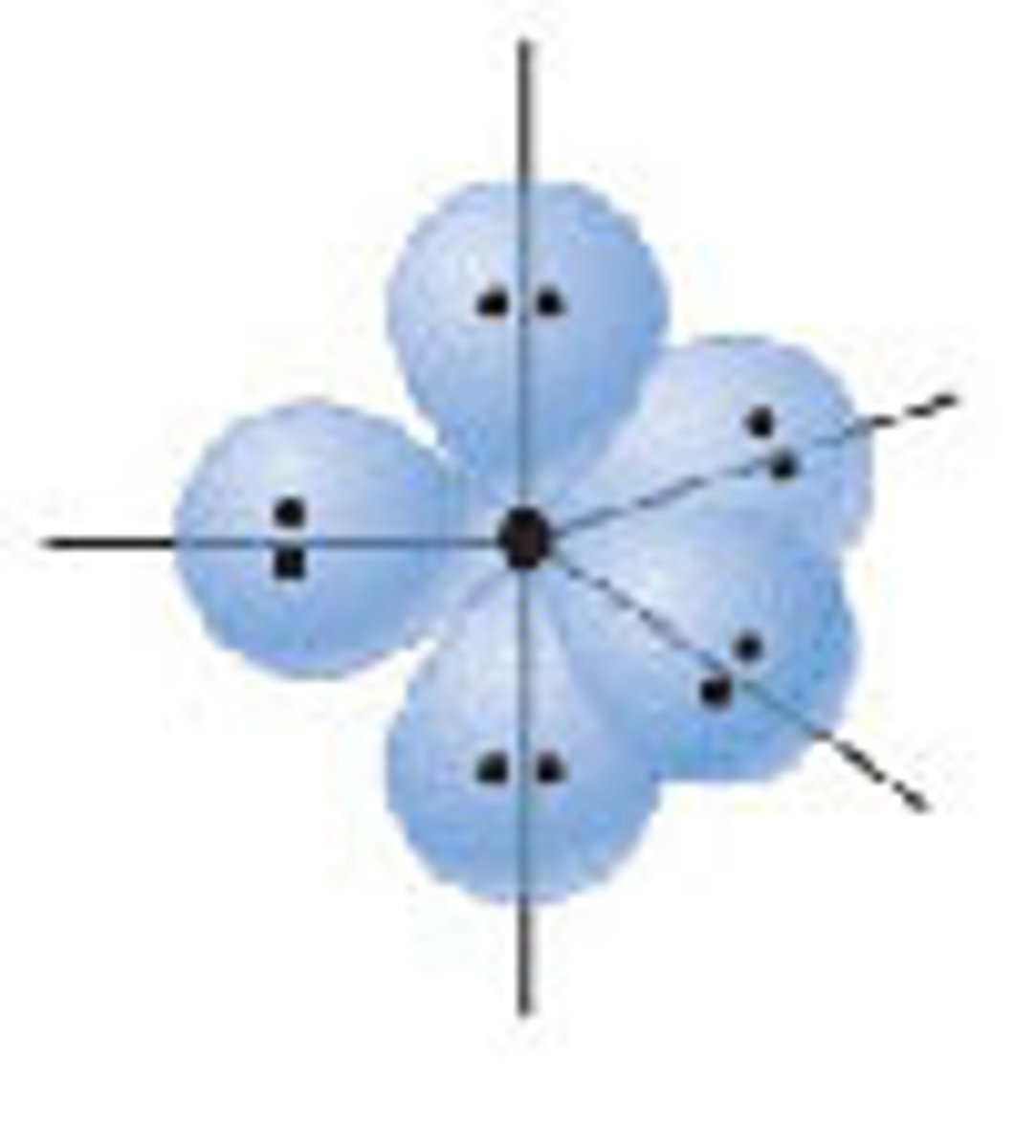
Diagram of Valence Theory hybridization orbital sp3d2: