Geometry and Hybridization
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
bent w/ 4 electron groups (bond angles)
<109.5 degrees
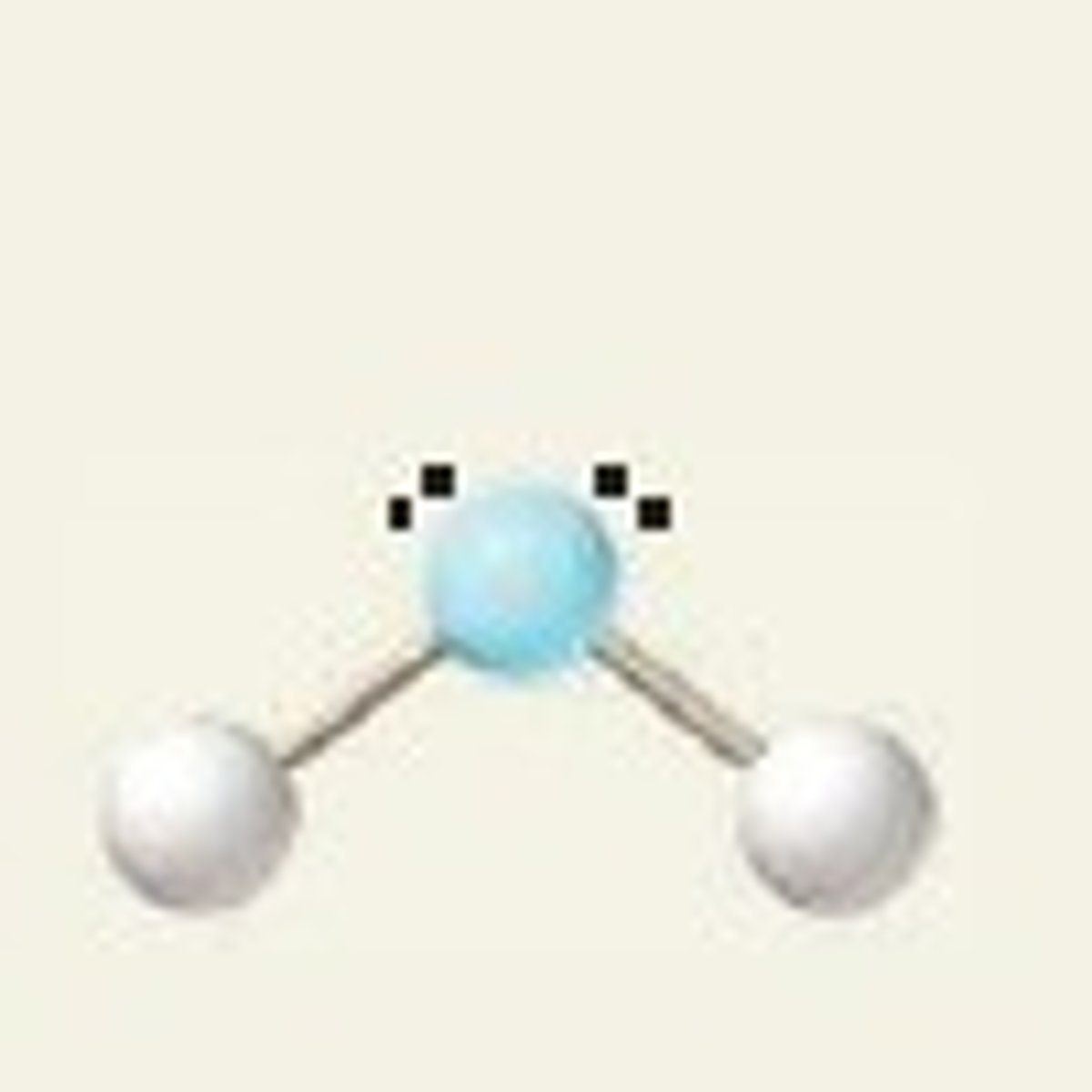
bent w/ 4 electron groups (hybridization)
sp^3
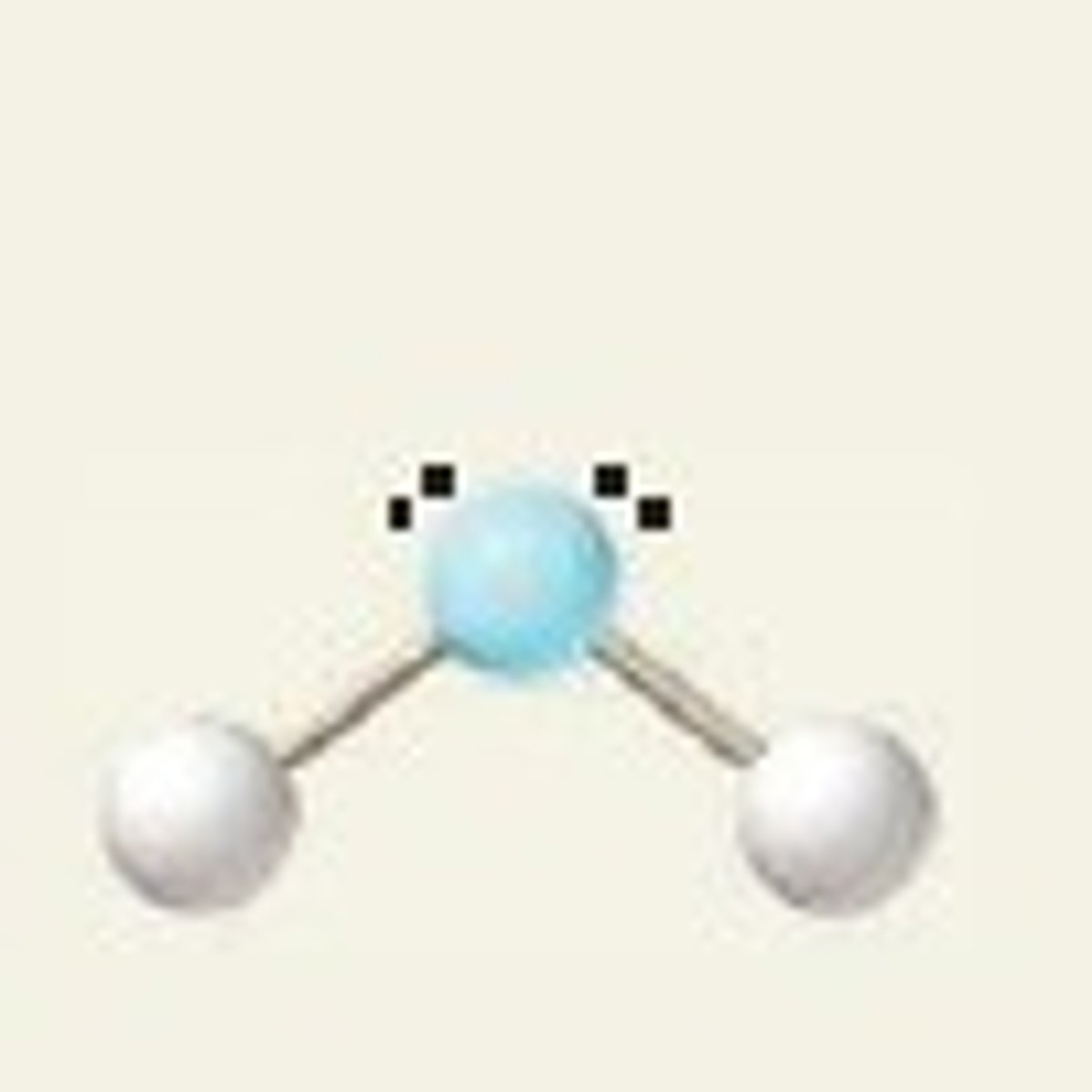
H₂O (molecular geometry)
bent
tetrahedral (bond angles)
109.5 degrees
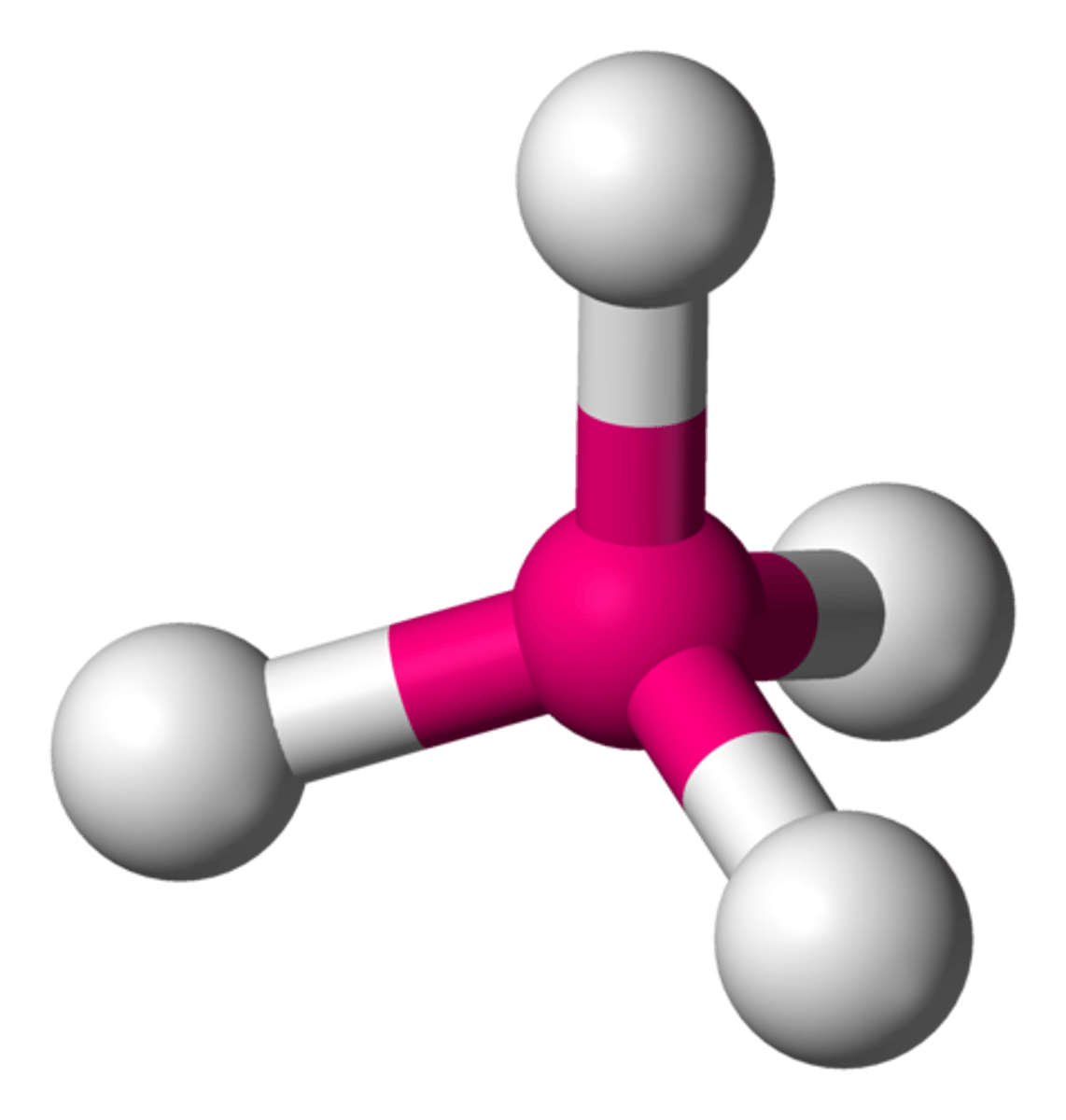
tetrahedral (hybridization)
sp^3
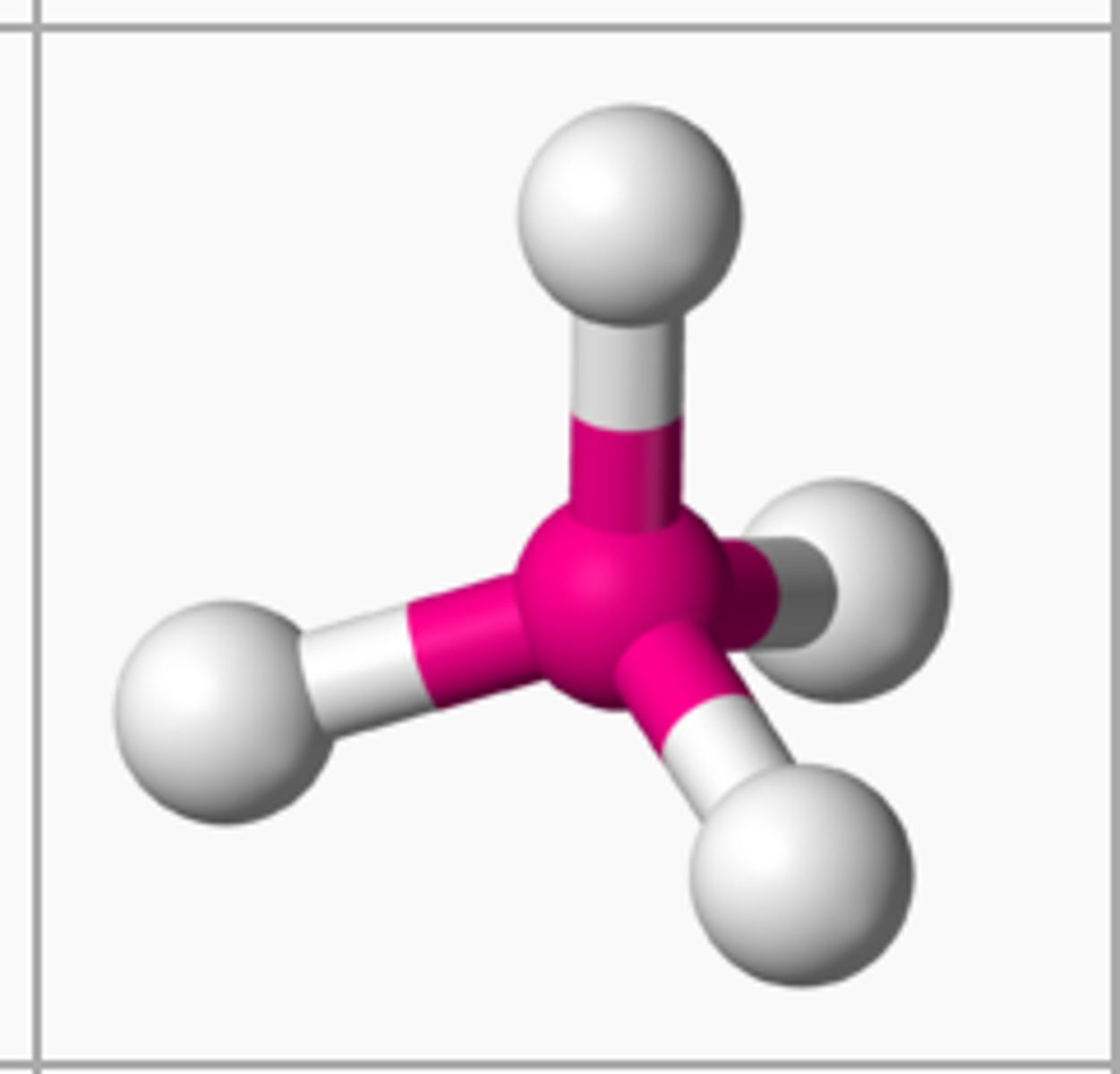
CH₄ (molecular geometry)
tetrahedral
Linear (bond angles)
180 degrees
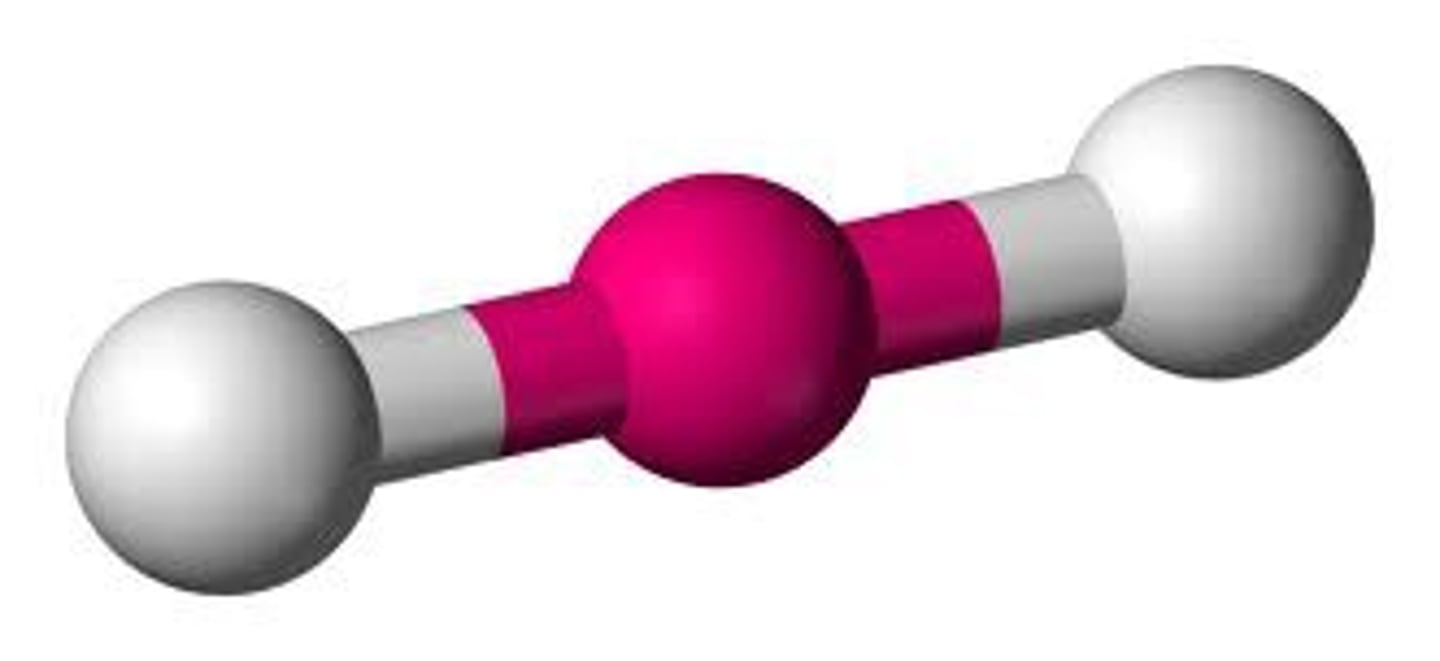
Linear (hybridization)
sp
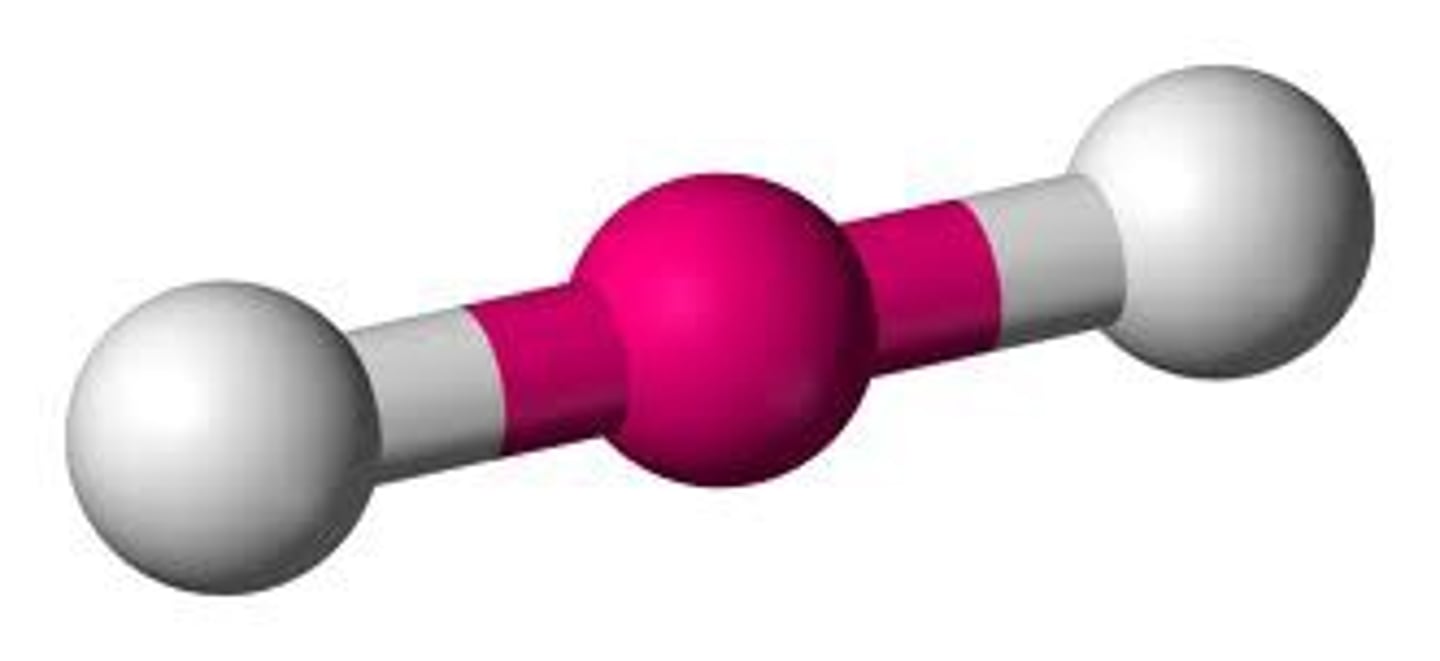
CO₂ (molecular geometry)
linear
trigonal pyramidal (bond angles)
<109.5 degrees
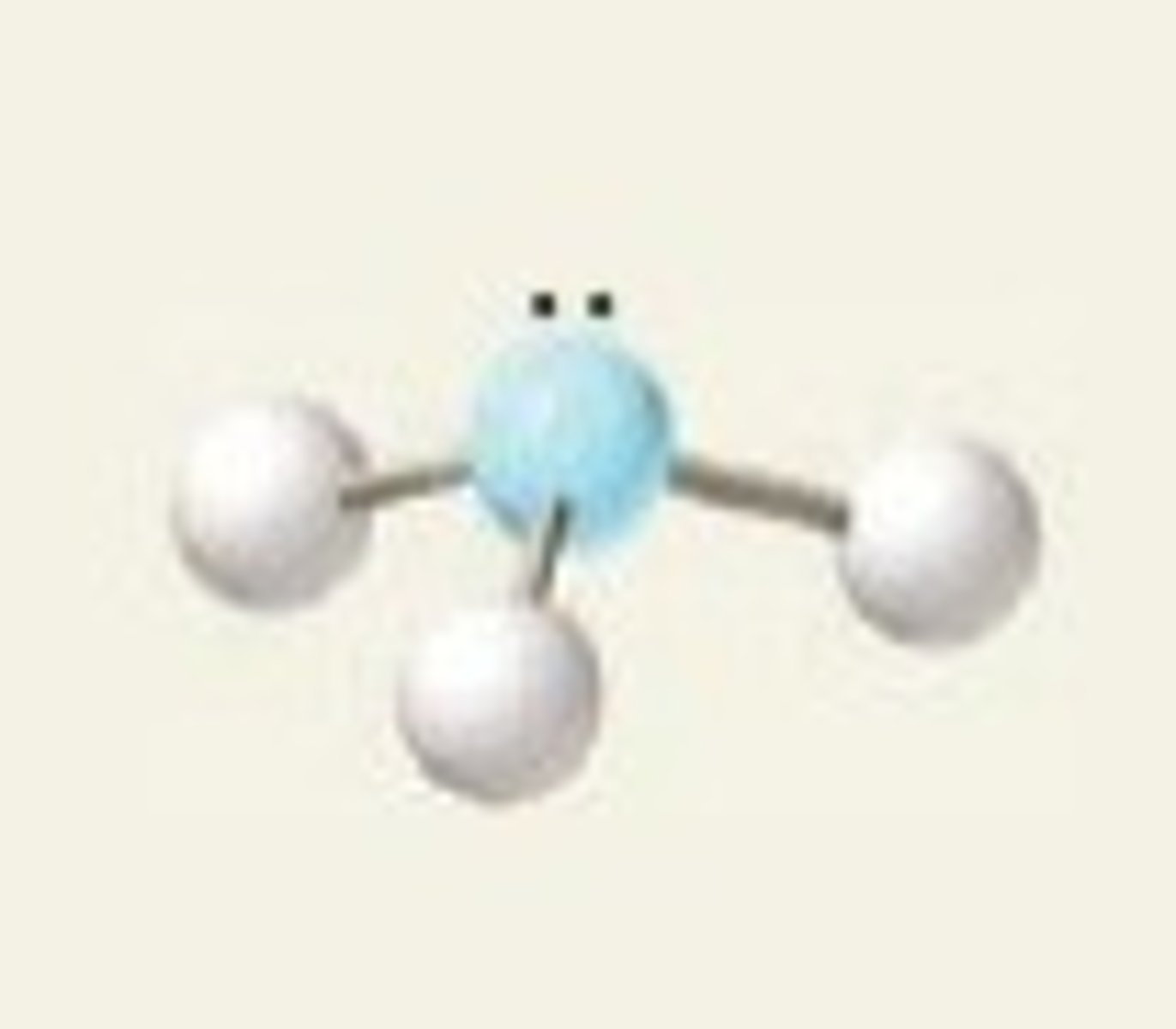
trigonal pyramidal (hybridization)
sp^3
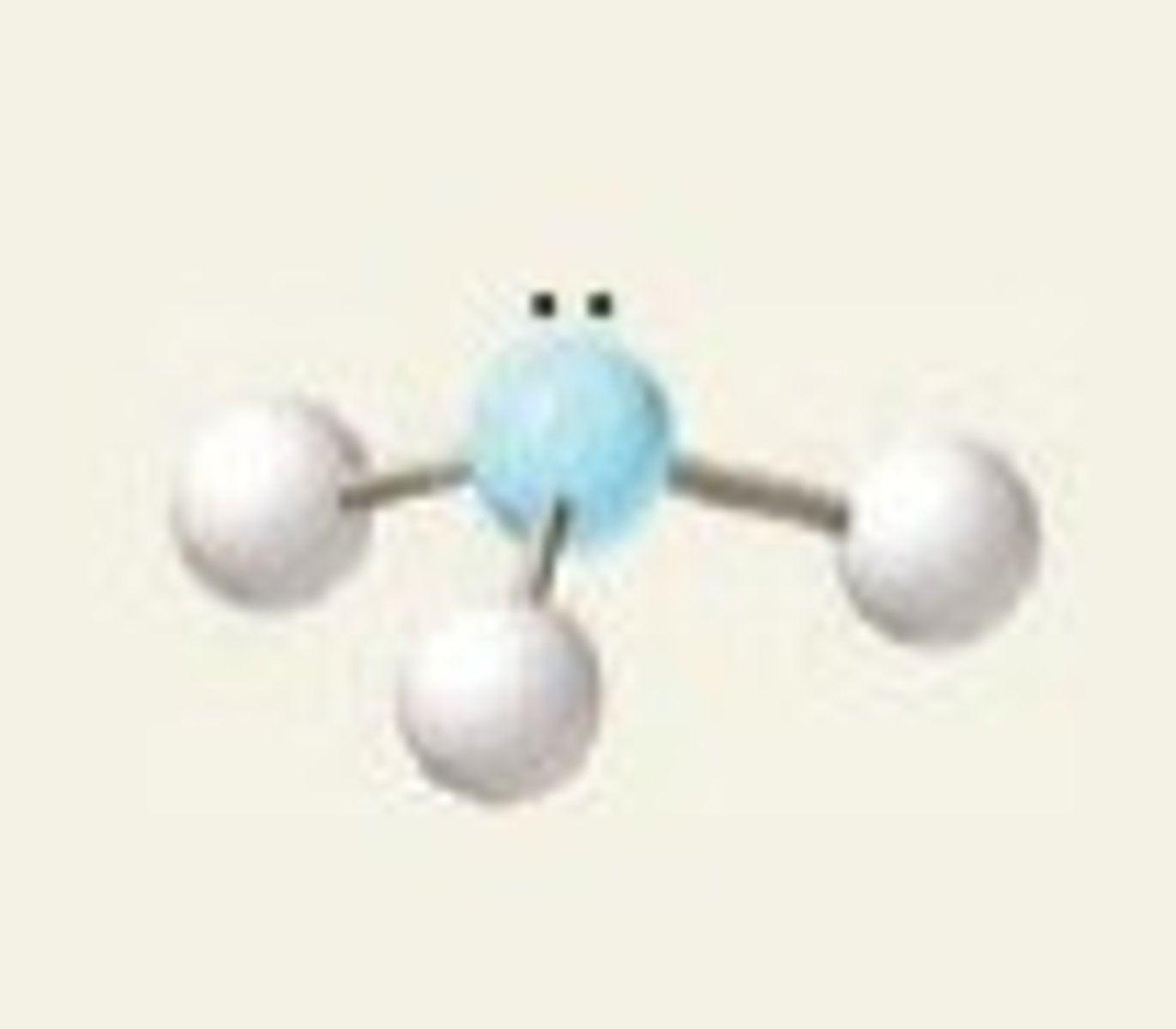
trigonal pyramidal (electron geometry)
tetrahedral
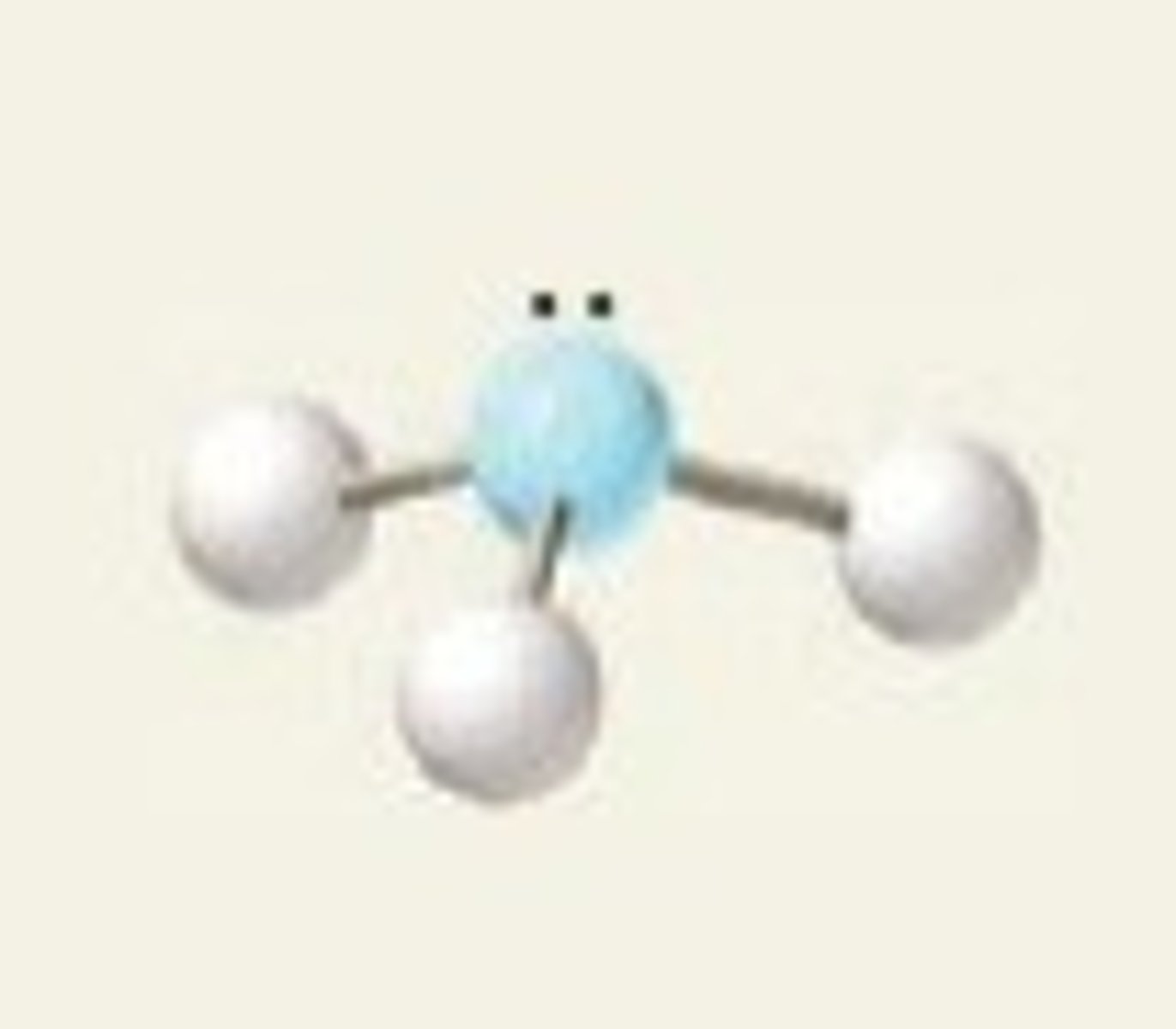
NH₃ (molecular geometry)
trigonal pyramidal
trigonal planar (bond angles)
120 degrees
trigonal planar (hybridization)
sp^2
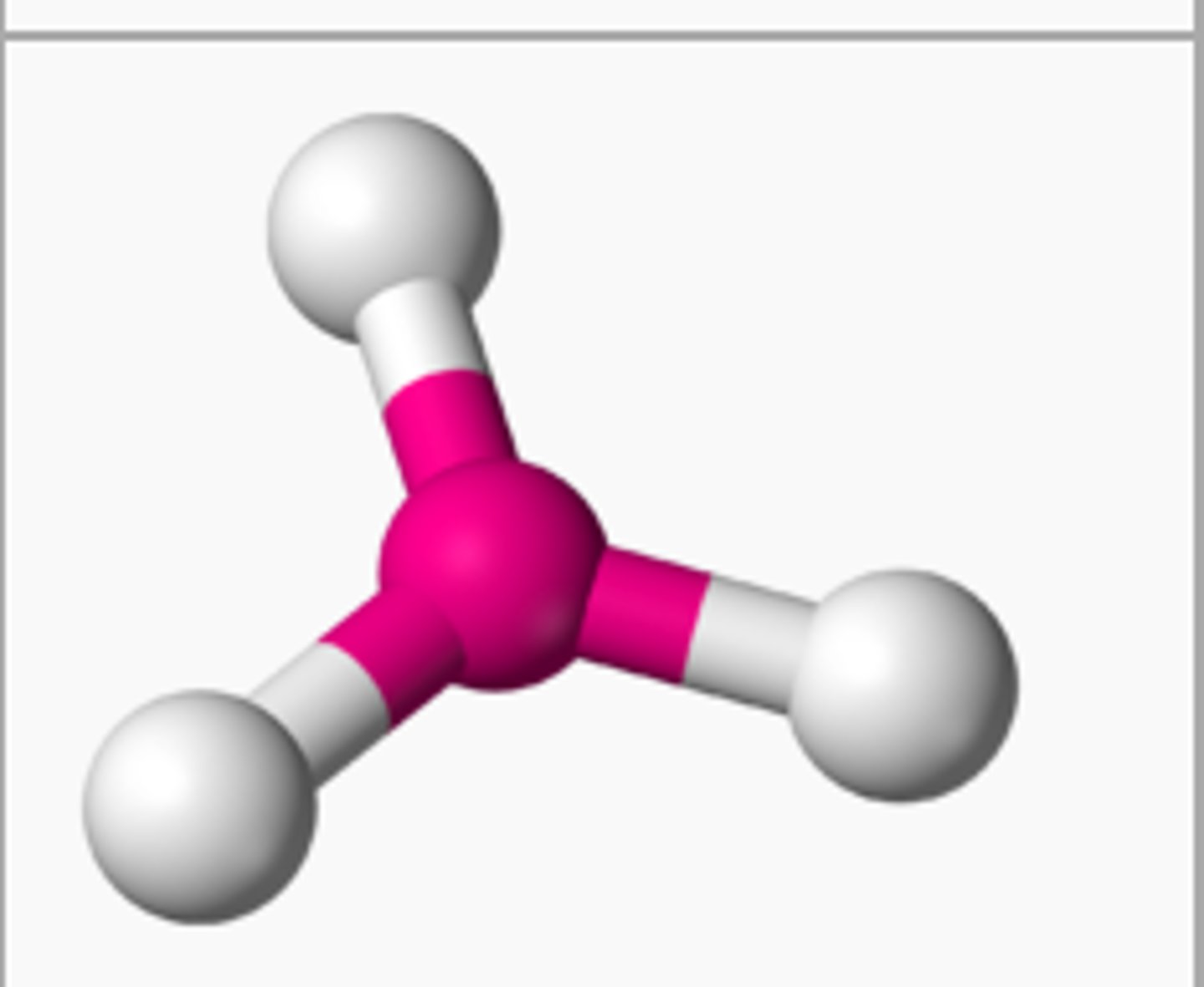
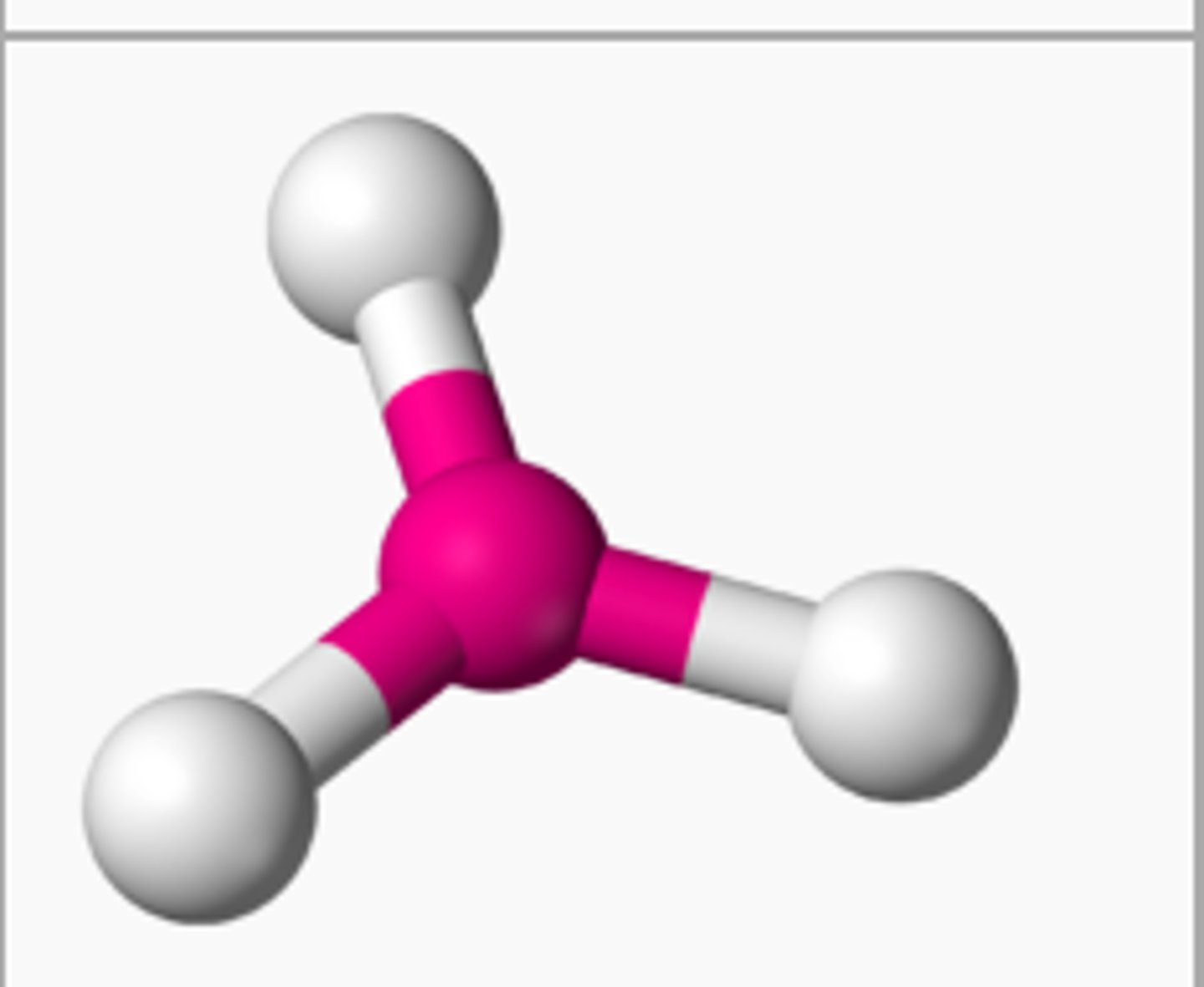
BF₃ (molecular geometry)
trigonal planar
trigonal bipyramidal (bond angles)
90 degrees & 120 degrees
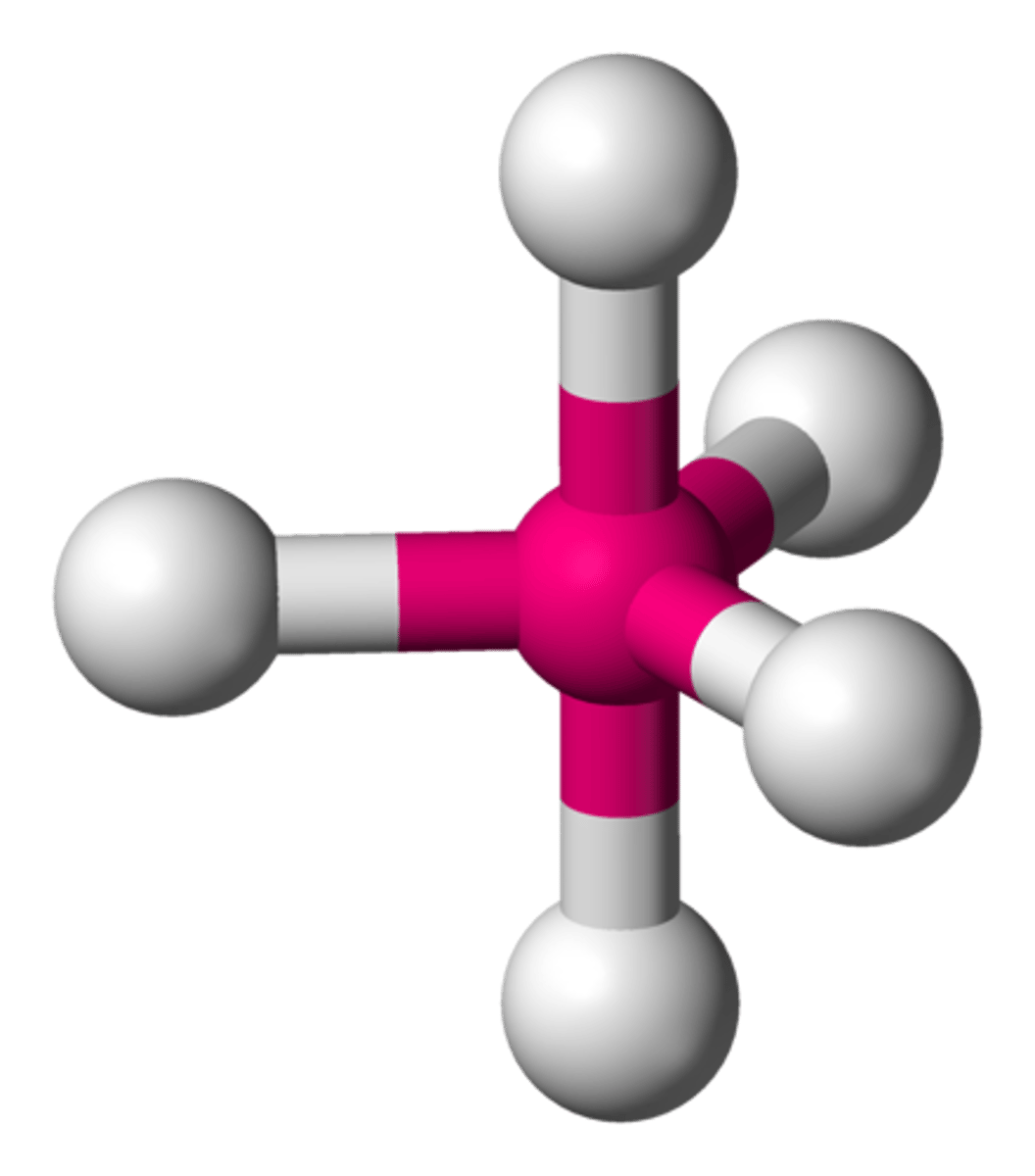
trigonal bipyramidal (hybridization)
sp^3d
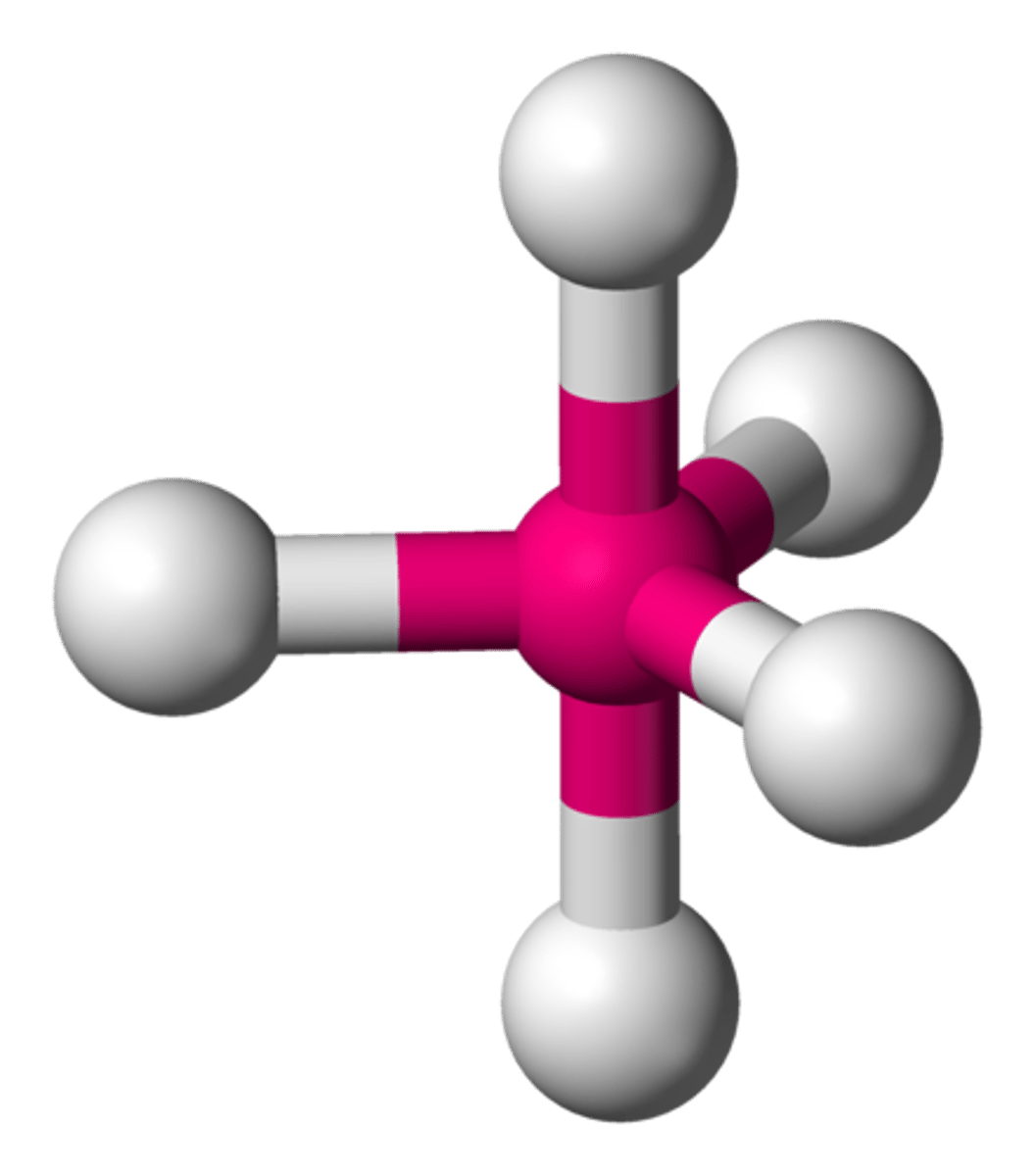
AsFCl₄ (molecular geometry)
trigonal bipyramidal
octahedral (bond angles)
90 degrees
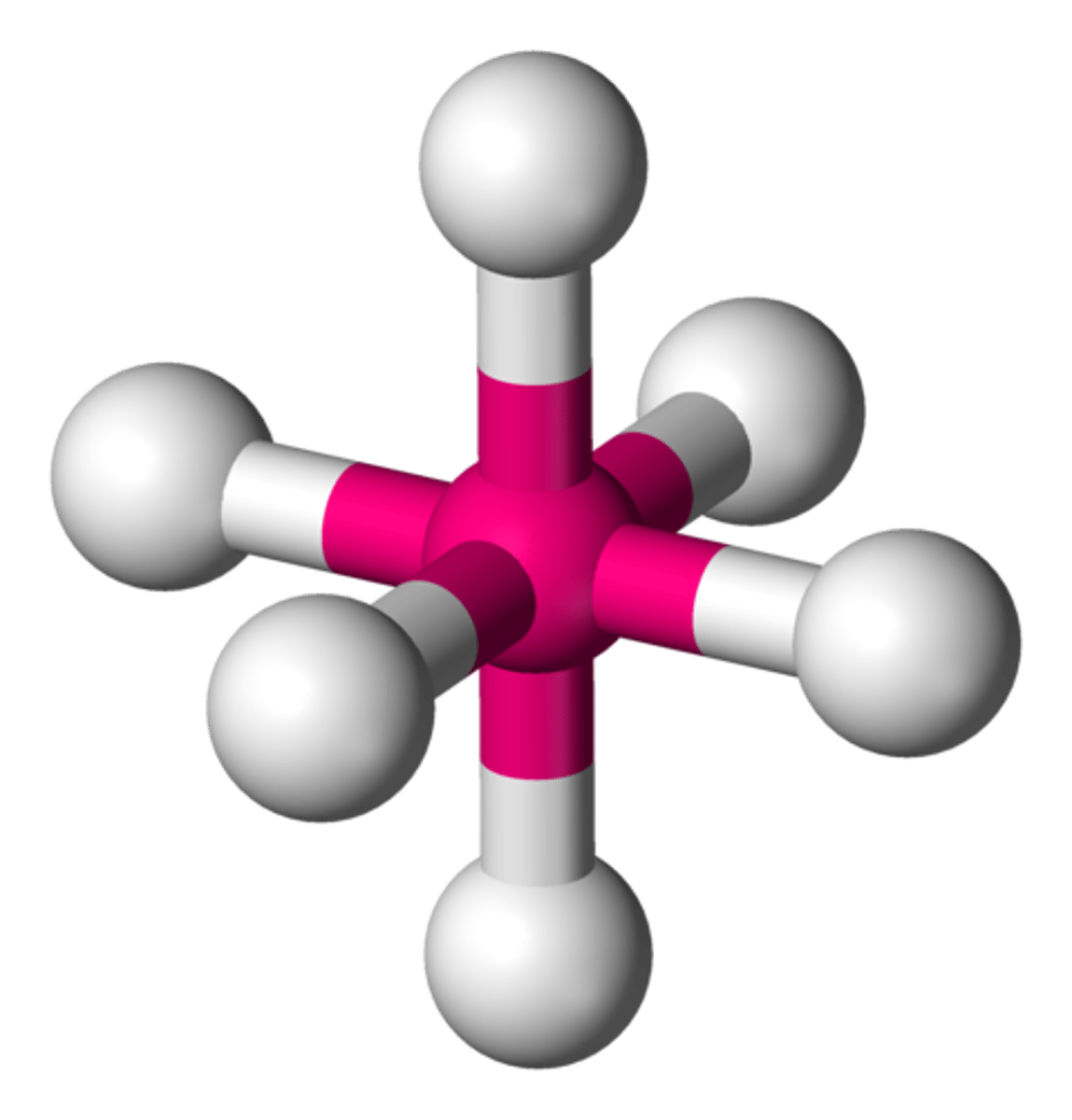
octahedral (hybridization)
sp^3d^2
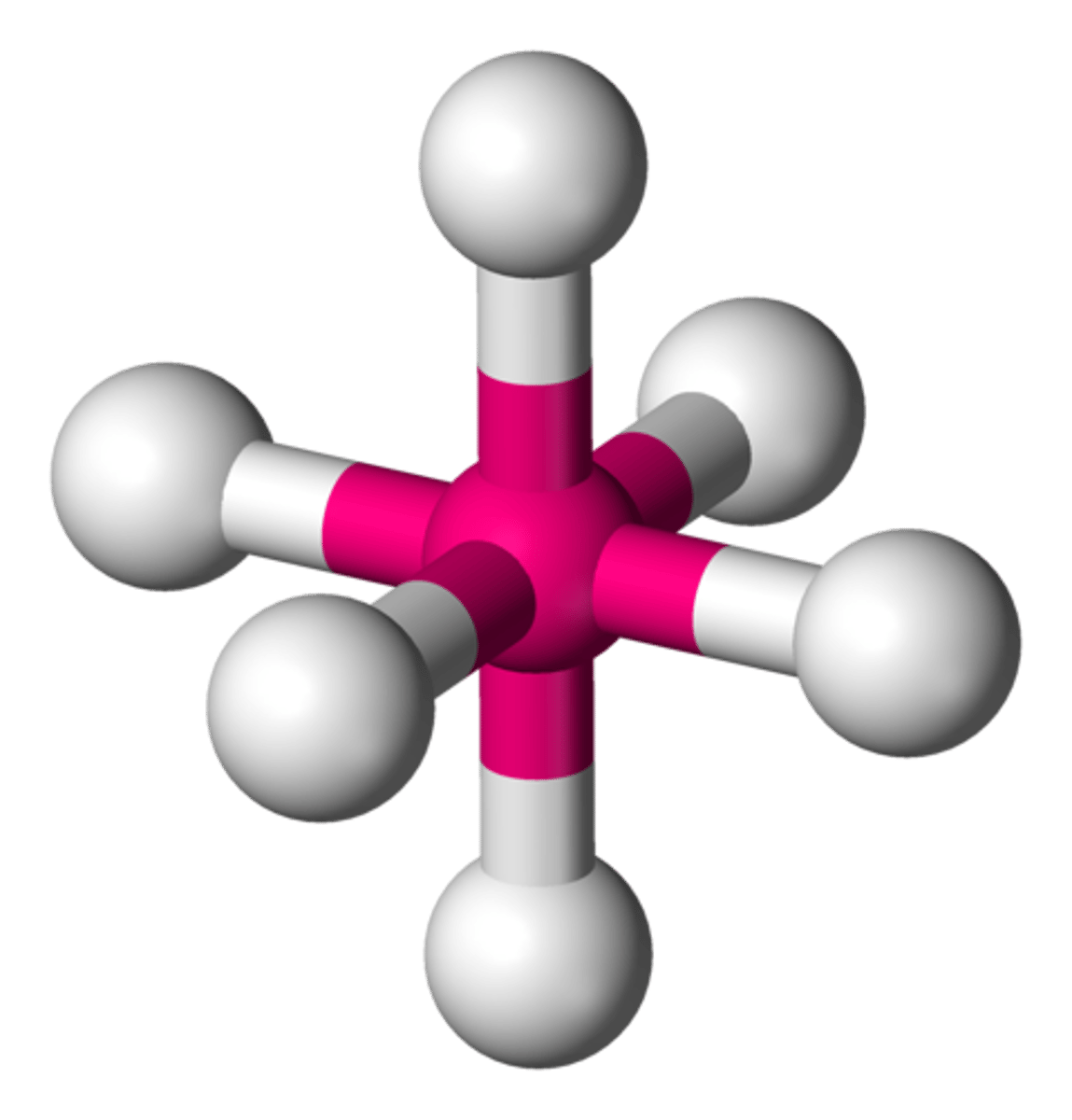
SF₆ (molecular geometry)
octahedral
see-saw (bond angles)
<90 degrees & <120 degrees
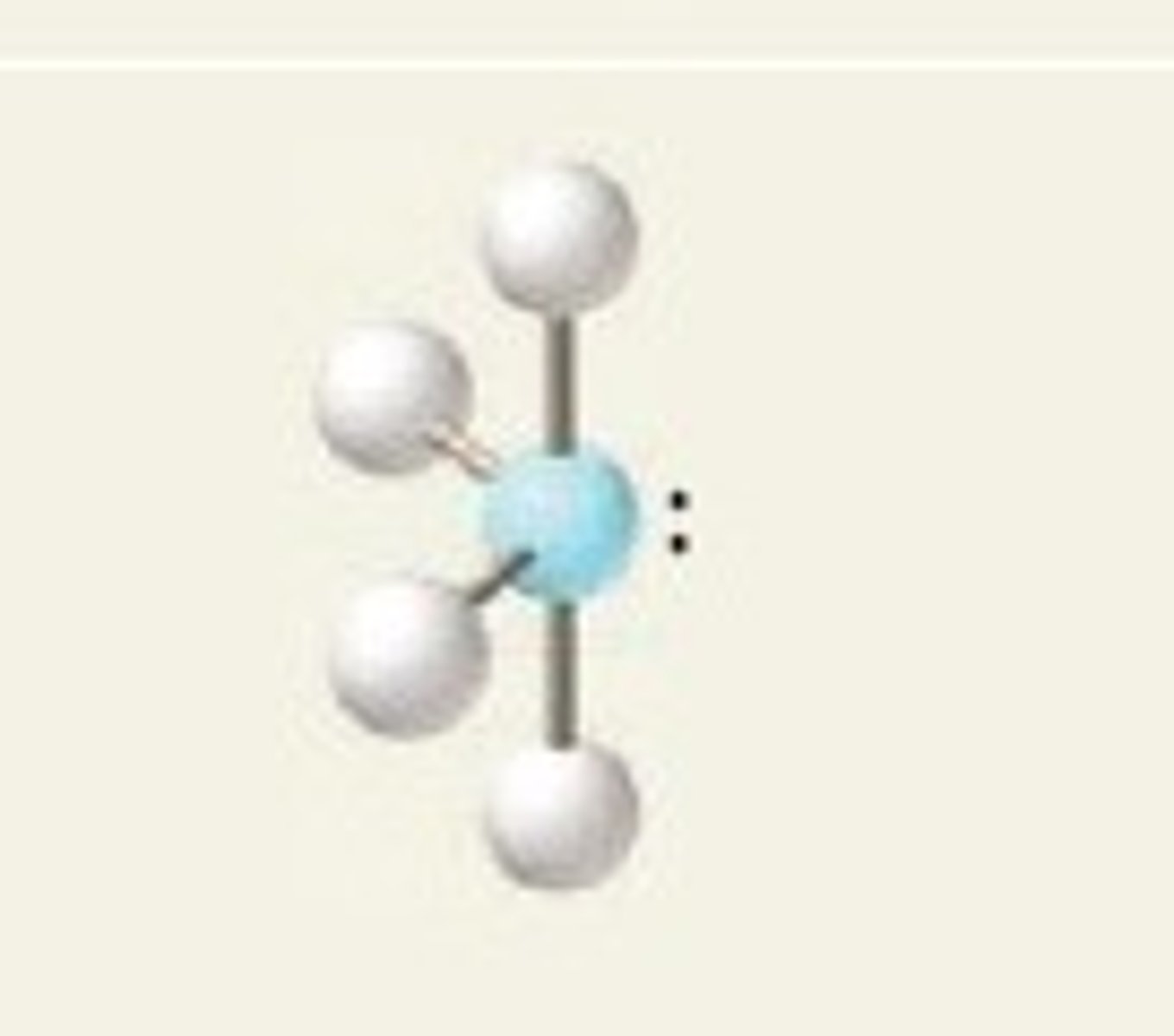
see-saw (hybridization)
sp^3d
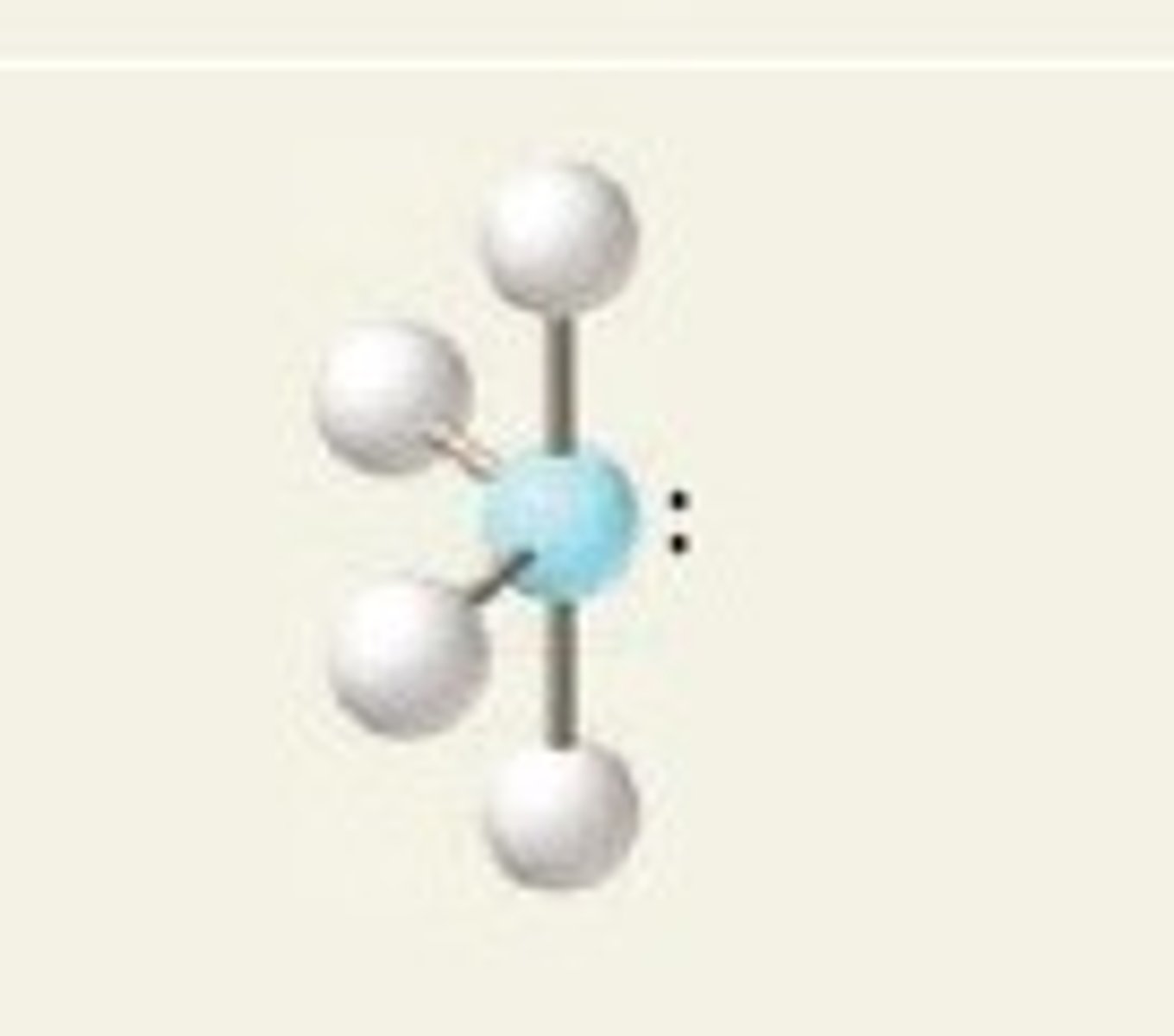
see-saw (electron geometry)
trigonal bipyramidal
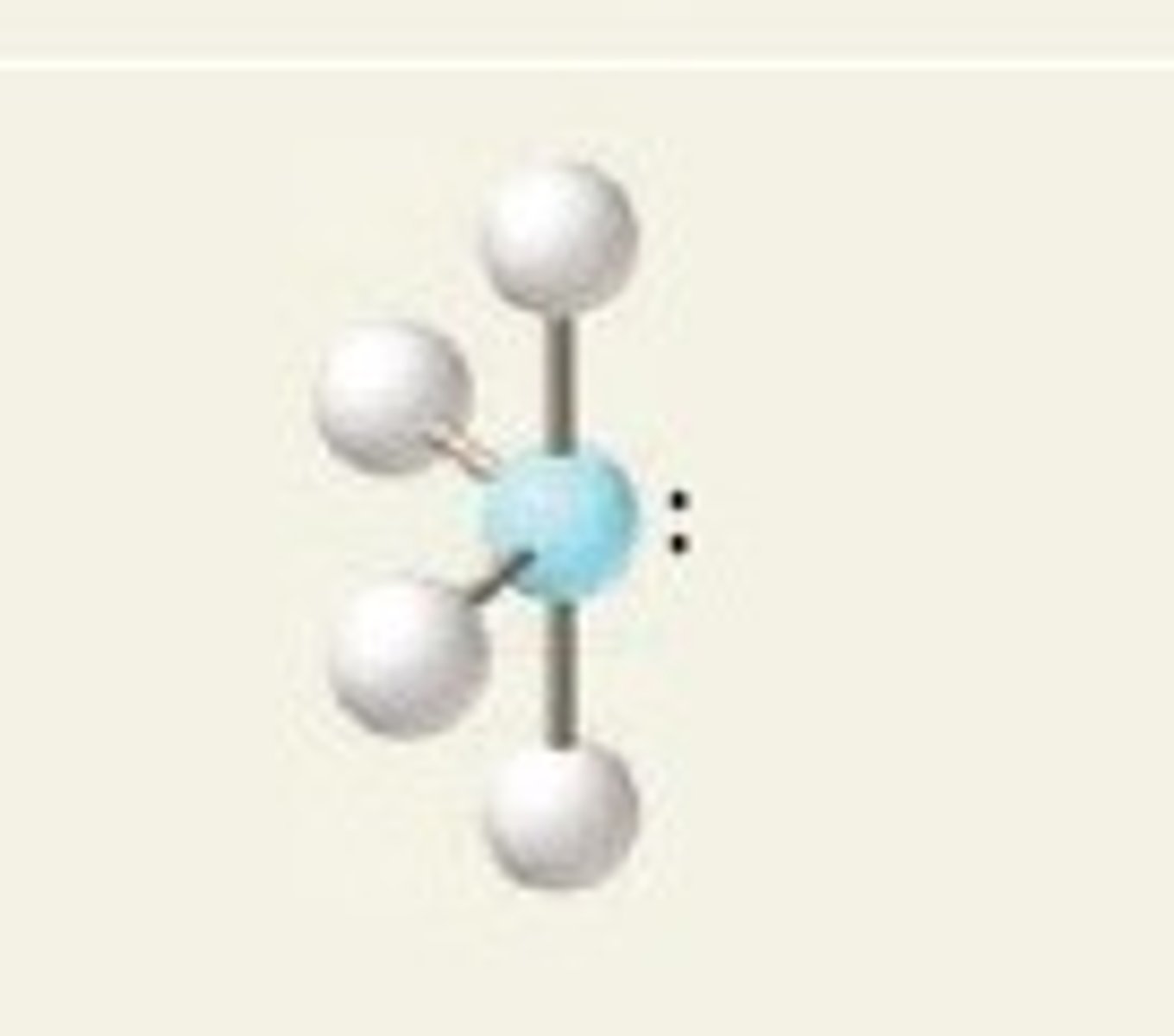
SF₄ (molecular geometry)
see-saw
T-shape (bond angles)
<90 degrees
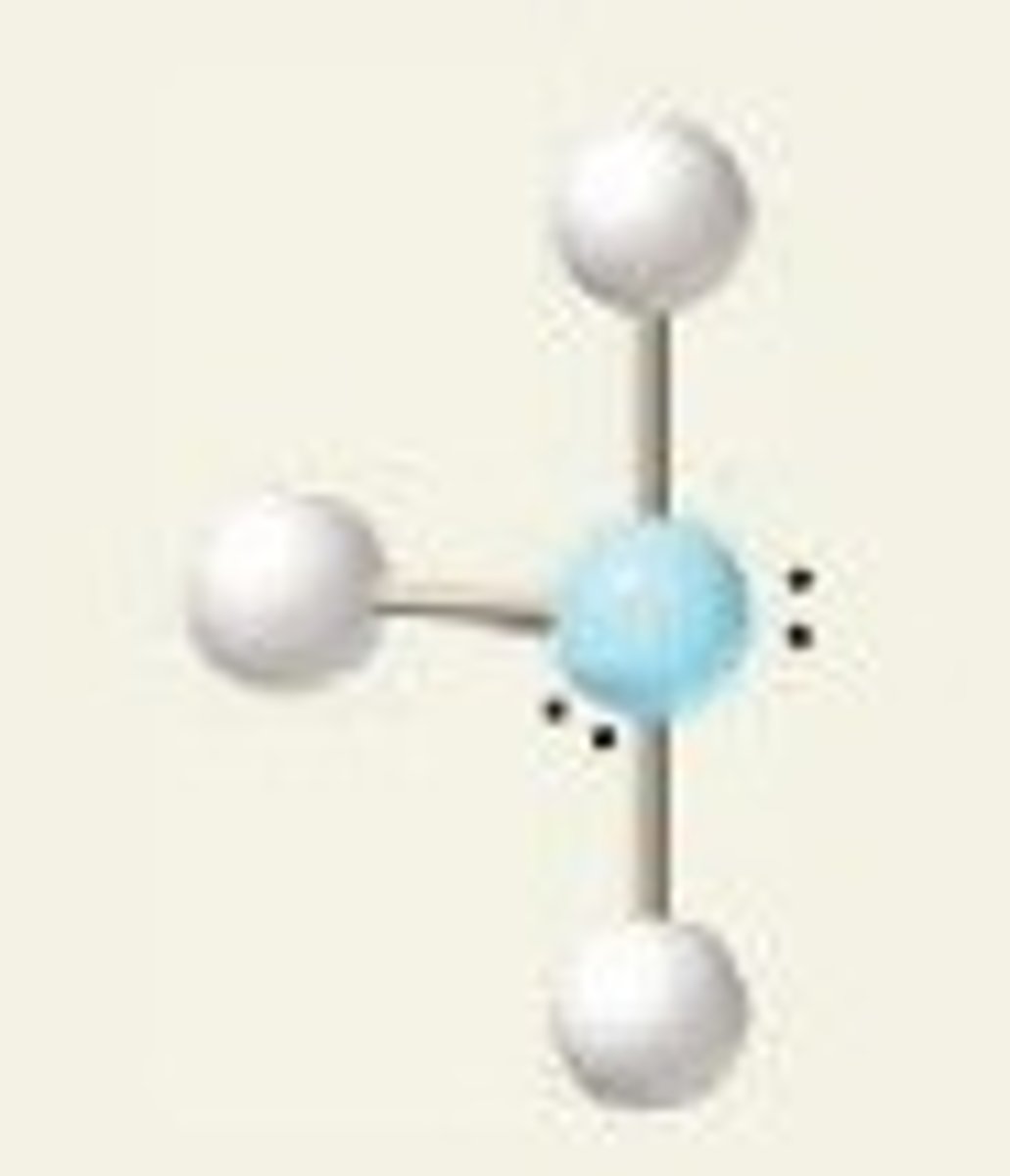
T-shape (hybridization)
sp^3d
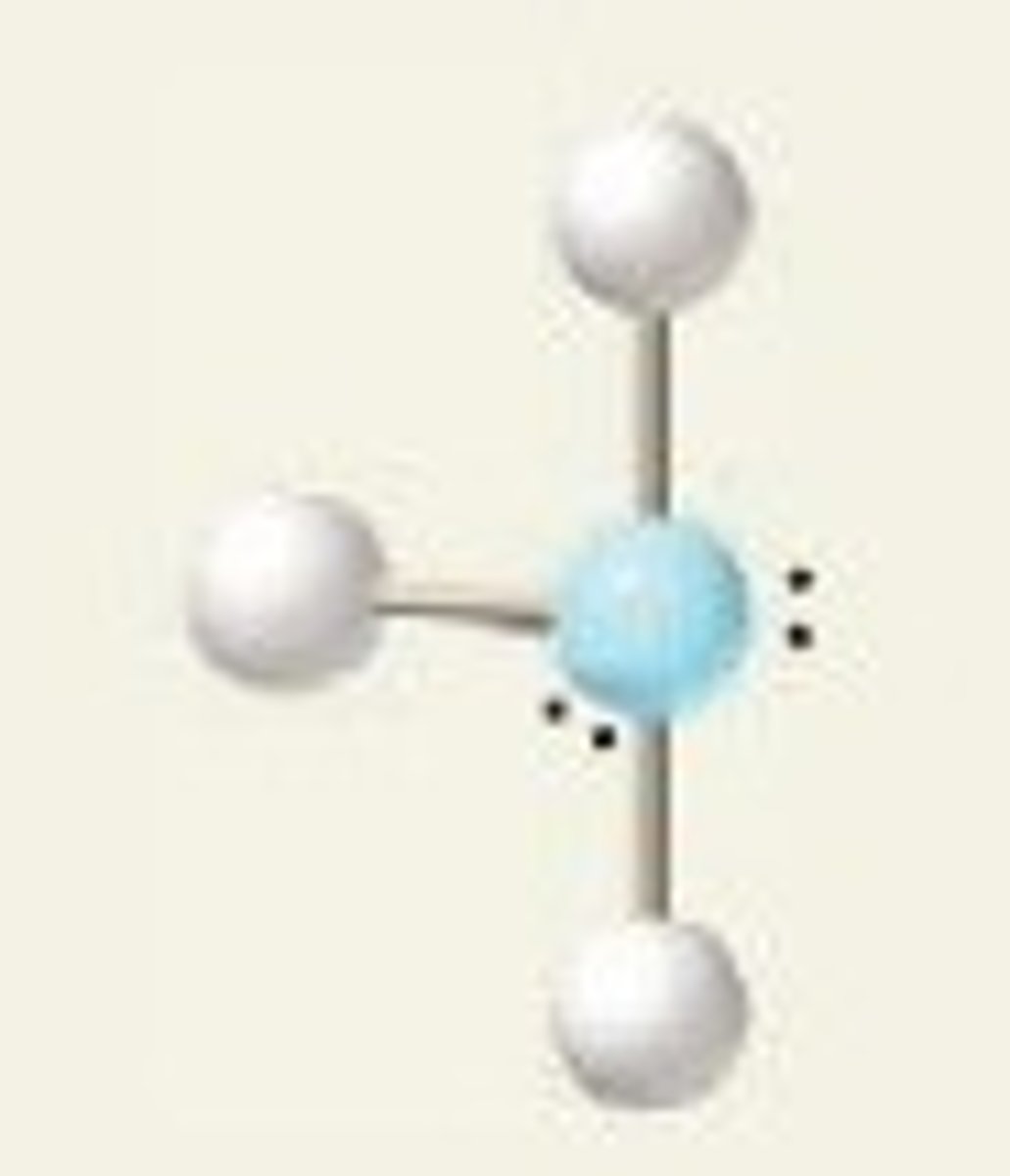
T-shape (electron geometry)
trigonal bipyramidal
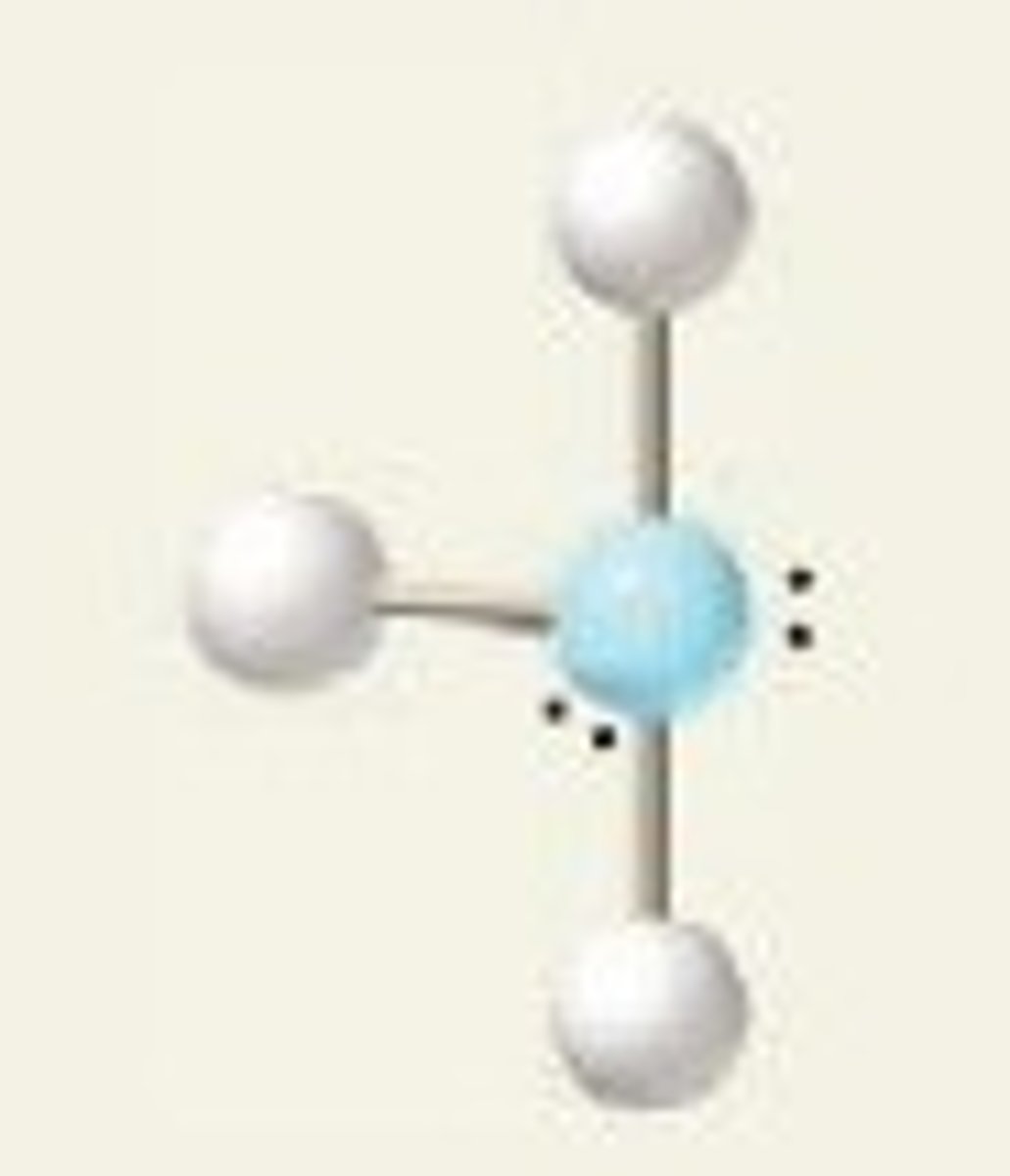
ClF₃ (molecular geometry)
T-shape
square pyramidal (bond angles)
<90 degrees
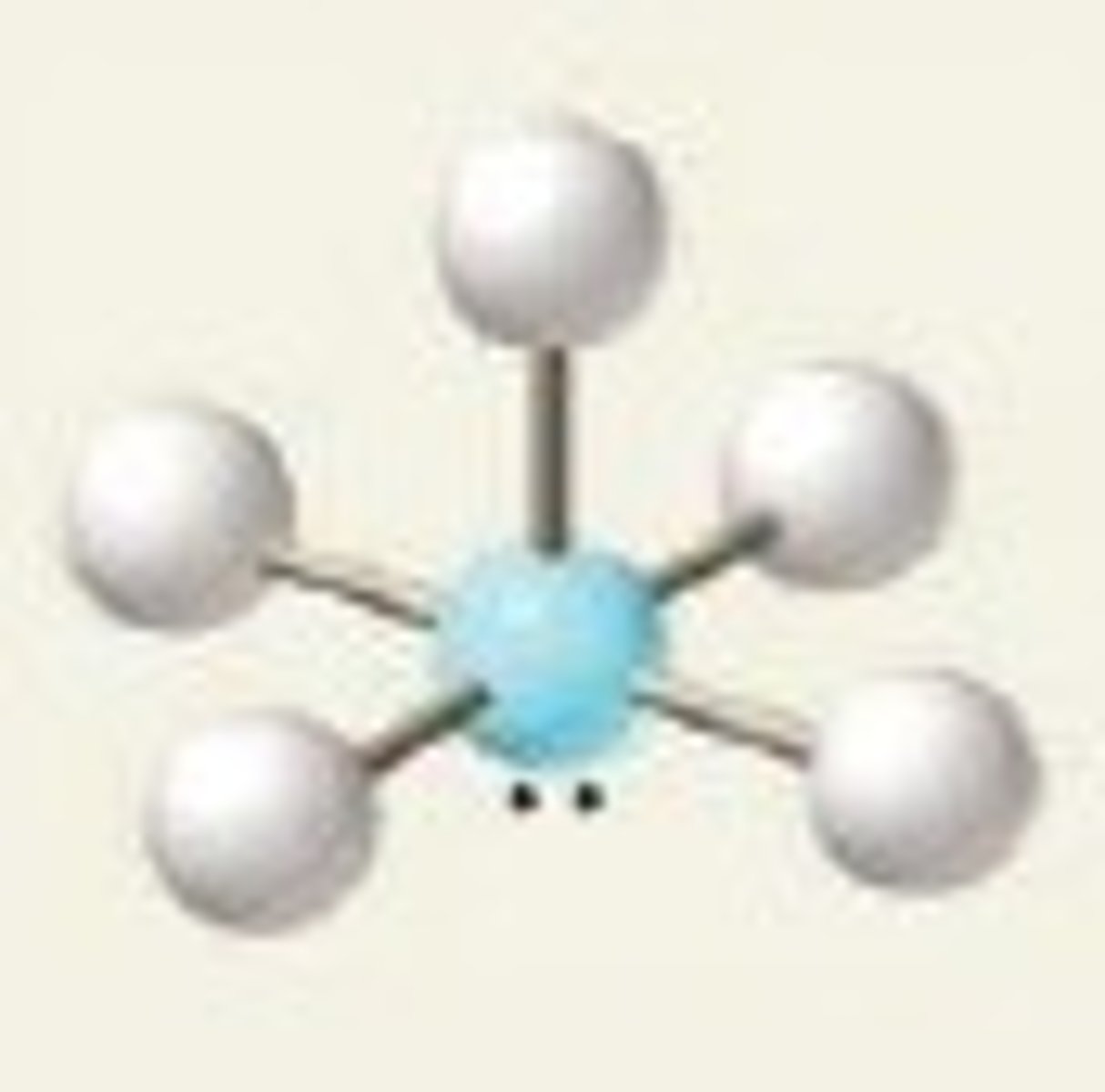
square pyramidal (hybridization)
sp^3d^2
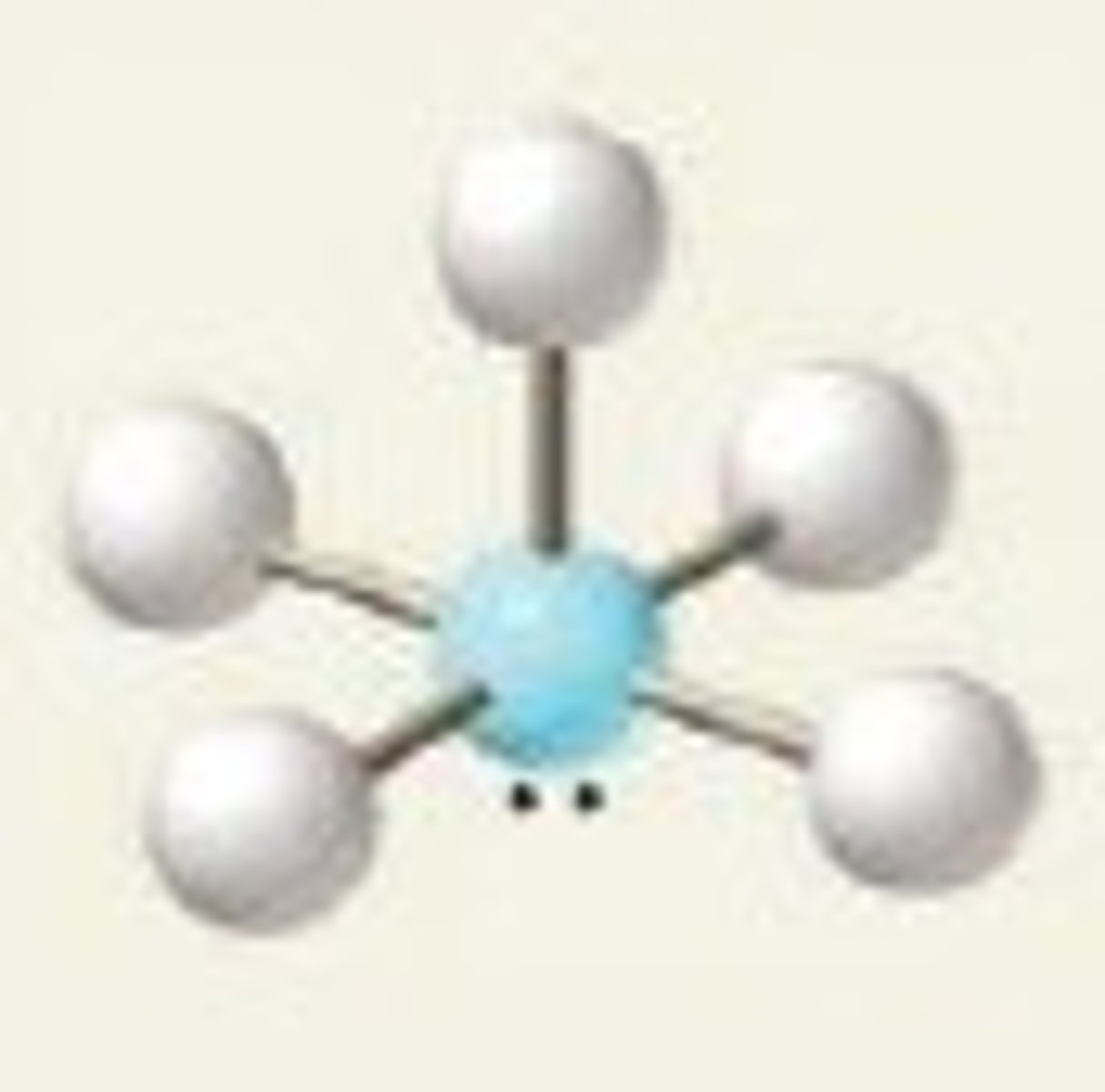
square pyramidal (electron geometry)
octahedral
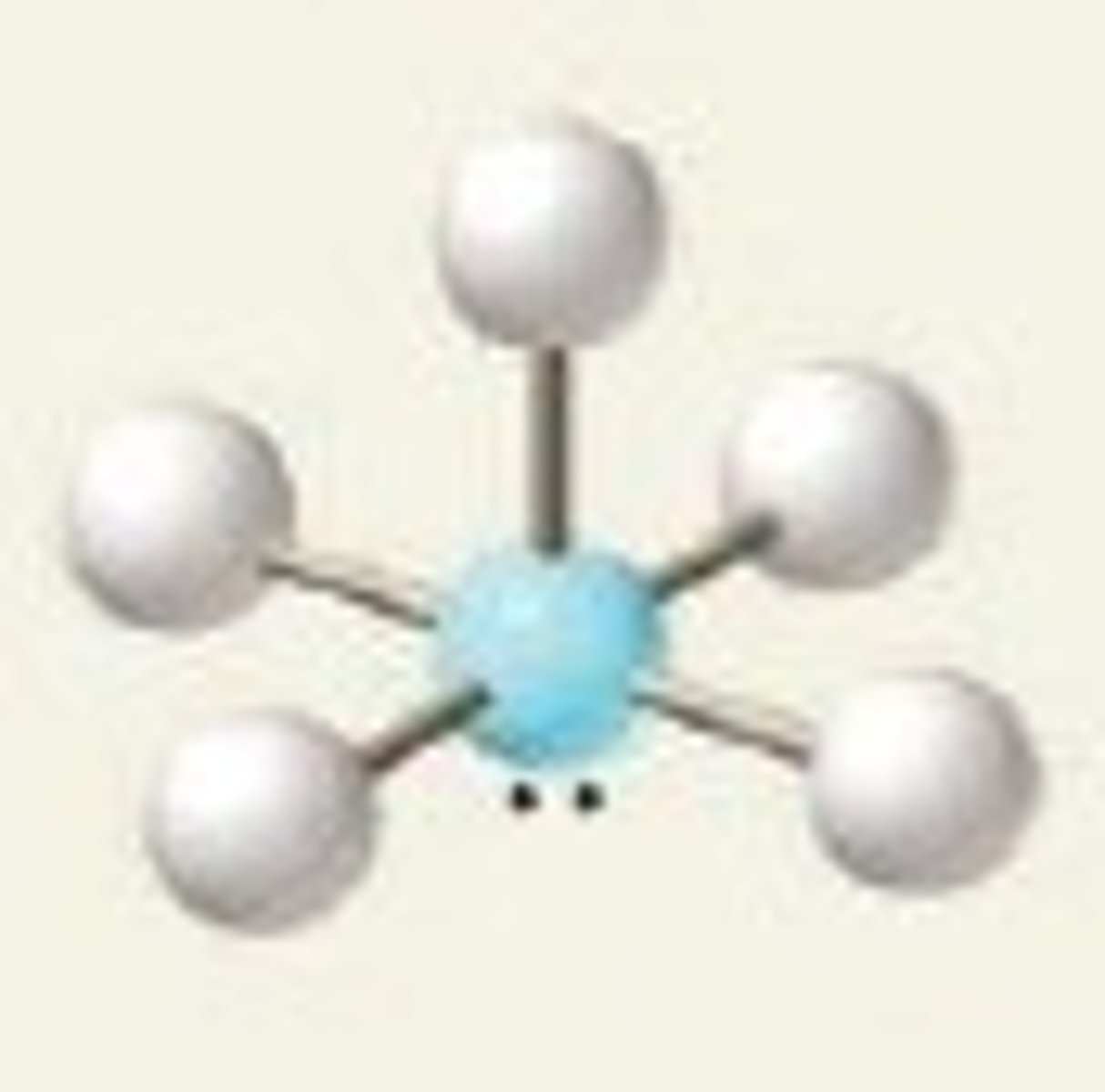
BrF₅ (molecular geometry)
square pyramidal
square planar (bond angles)
90 degrees
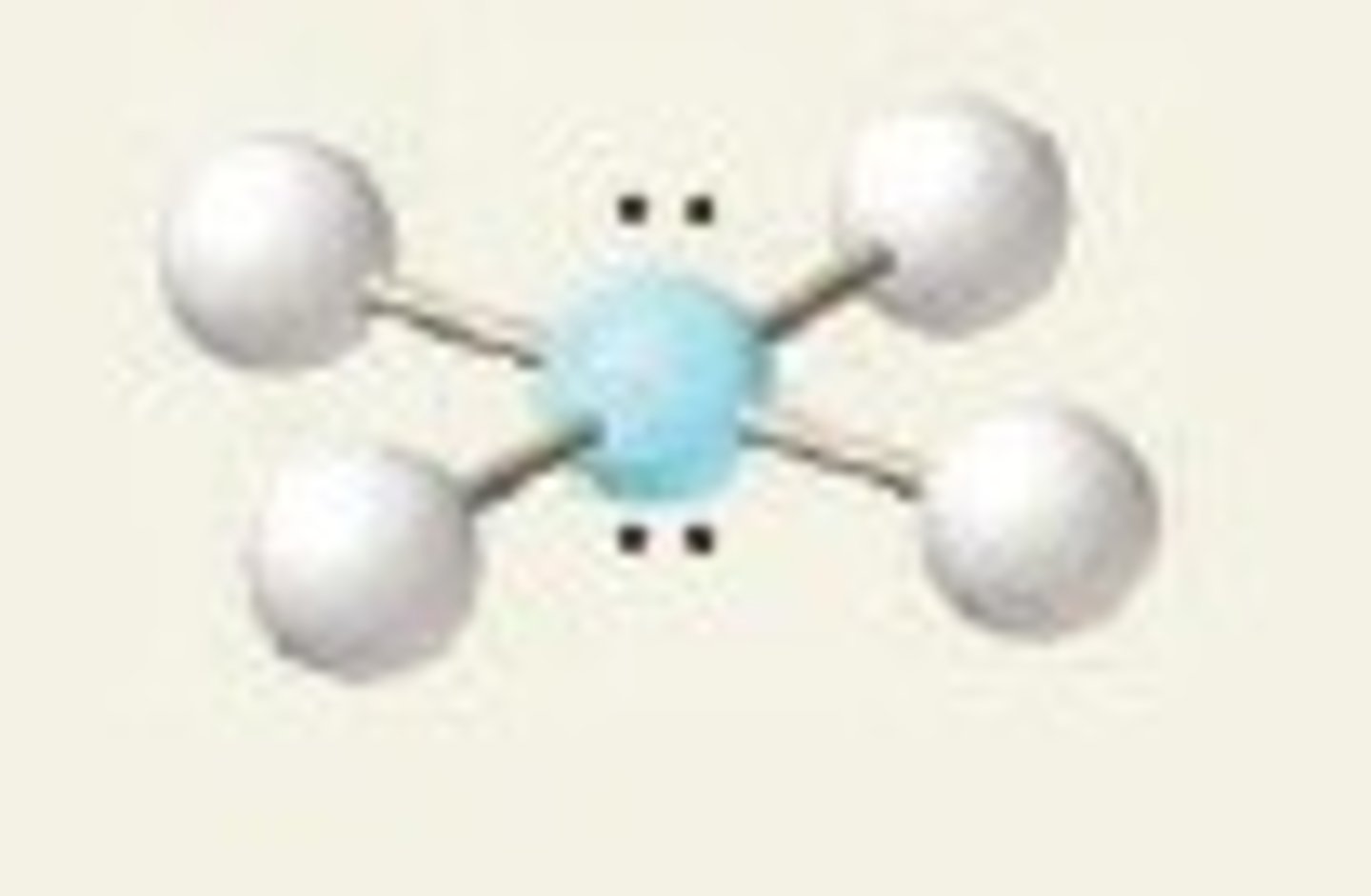
square planar (hybridization)
sp^3d^2
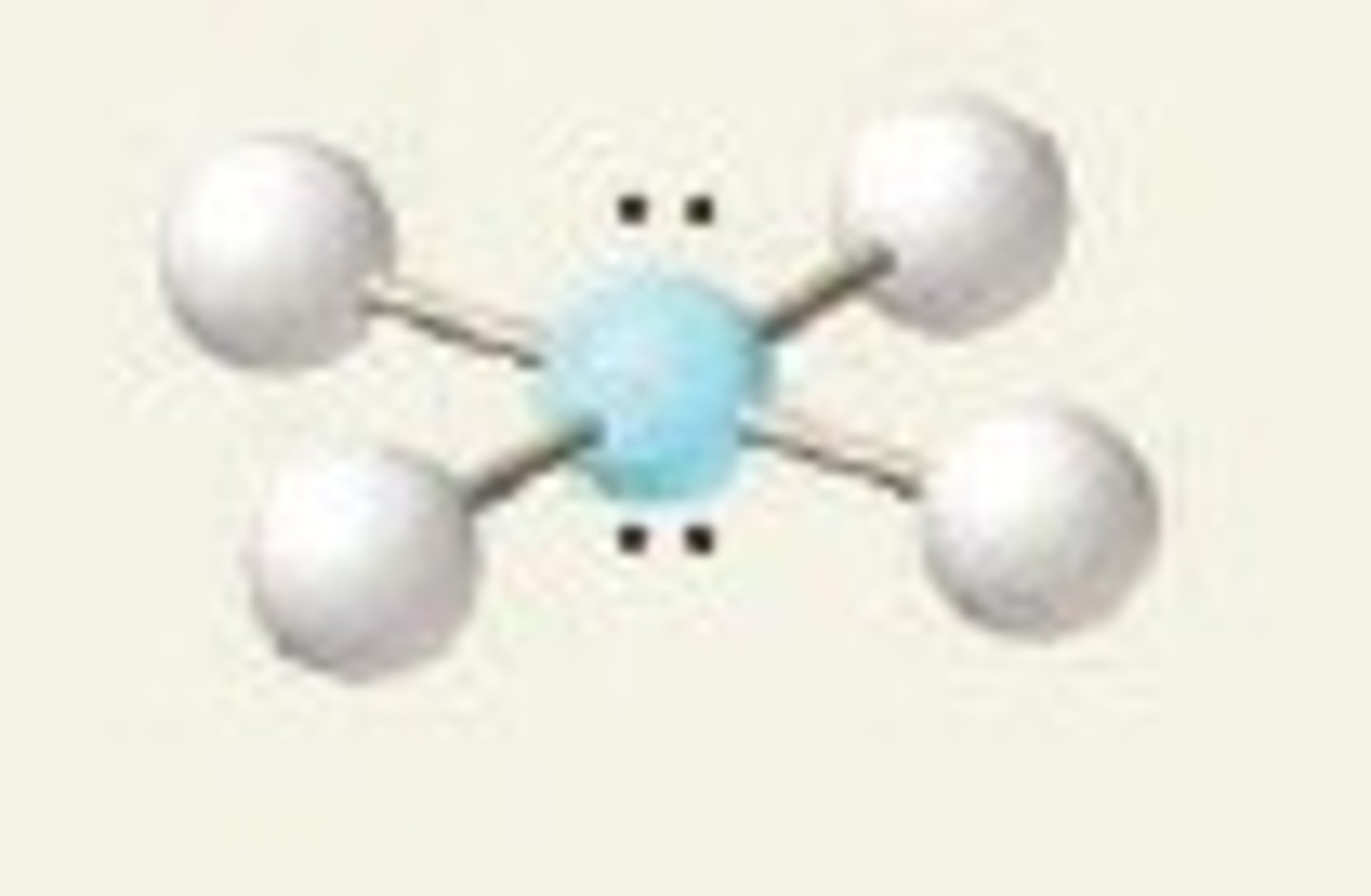
square planar (electron geometry)
octahedral
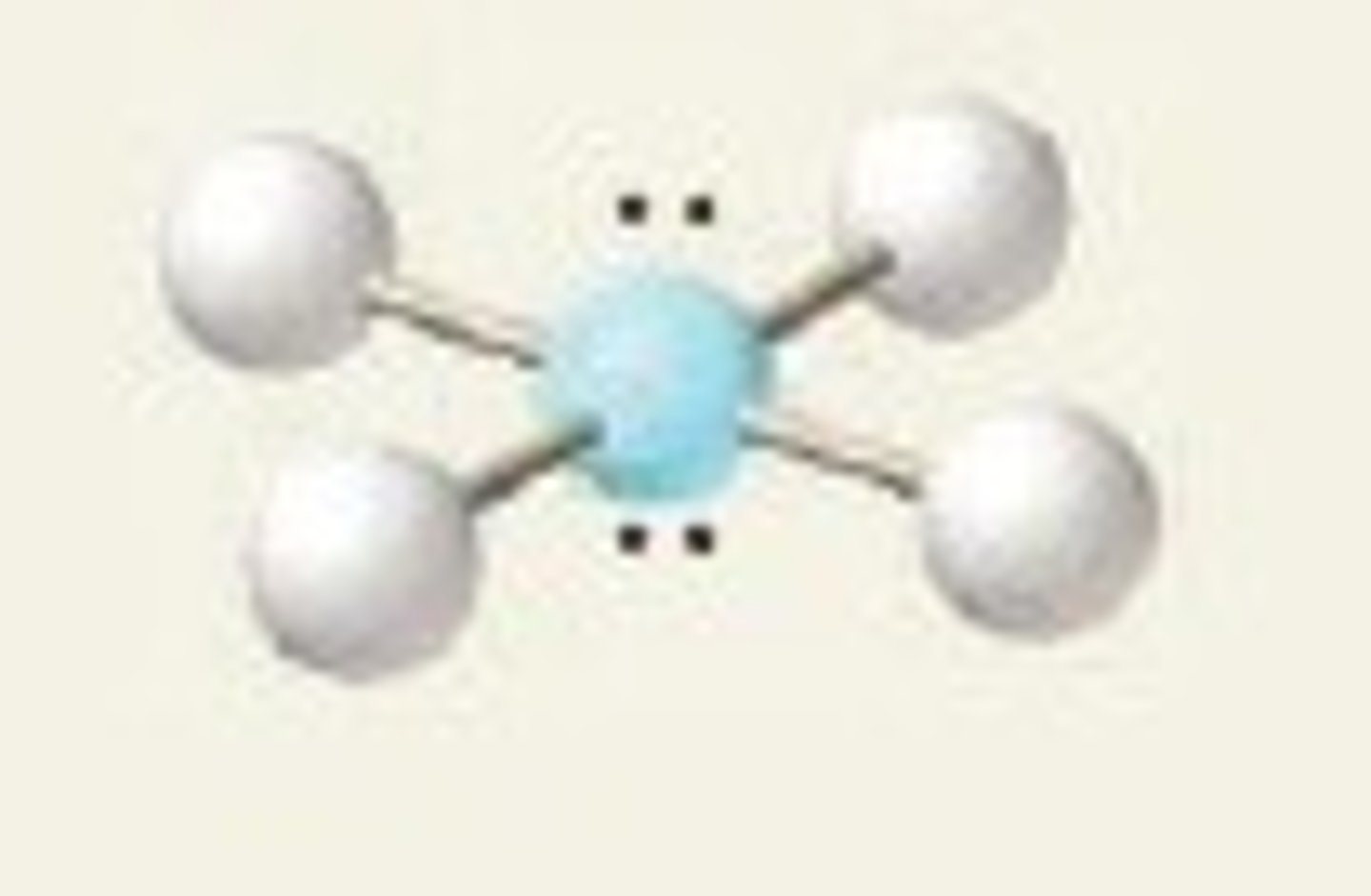
XeF₄ (molecular geometry)
square planar