Models to materials
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
bonding models
bonding types can be used to explain chemical and physical properties of substances, but these have limitations
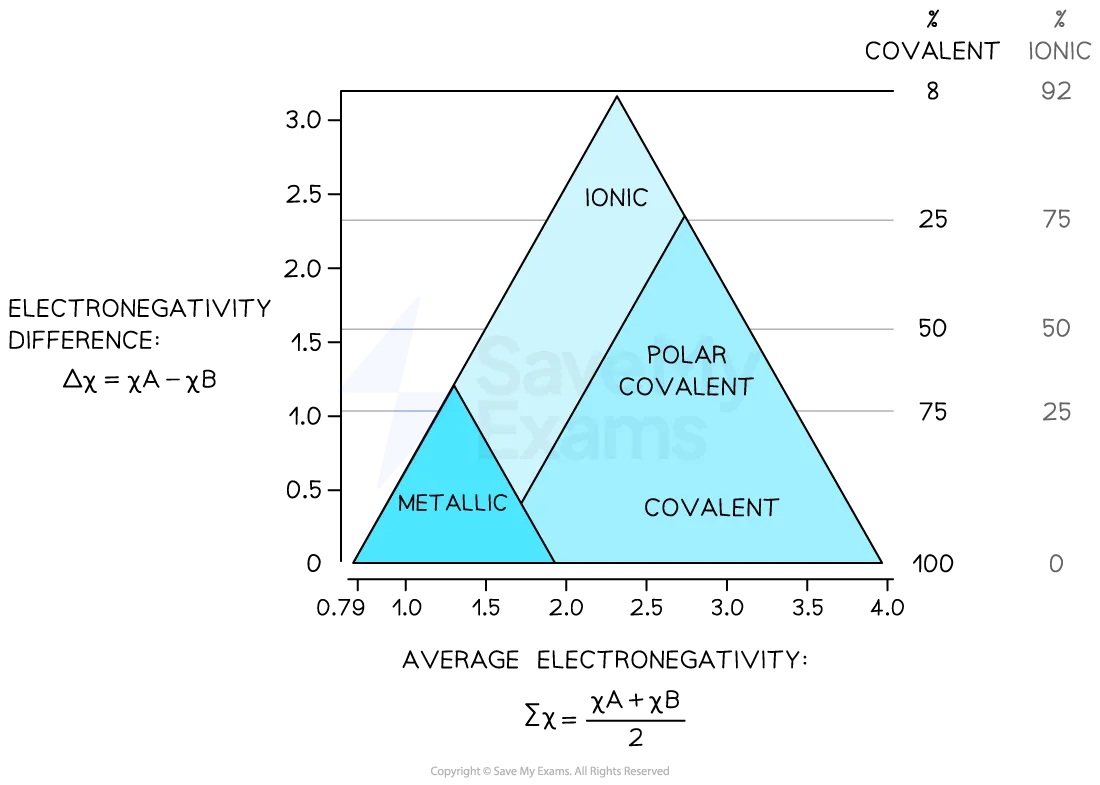
bonding is best thought of as a continuum of the three different bonding types like the area of an equilateral triangle
bonding triangle
location of an element or a compound on the bonding triangle is determined by electronegativity values of the elements present
elements have 0 difference in electronegativity so would be found along the x-axis
ionic compounds have a large diff, so would be found at the top
covalent bonds have a small diff, so are found at the bottom
alloys
an alloy is a mixture of metals, where the metals are mixed together physically but not chemically
they can also be made of metals mixed with non-metals
ions of different metals are spread throughout the lattice and are bound together by delocalised electrons
it’s possible to form alloys due to the non-directional nature of metallic bonds
why do alloys have different properties to pure metals
alloys have distinct properties due to different packing of cations in the lattice
they have properties that can be very different to the metals they contain
alloys contain atoms of different sizes, which distorts the regular arrangement of cations
makes it more difficult for the layers to slide over eachother, so they’re usually harder than the pure metal
examples of alloys
brass
copper & zinc
strong and resistant to corrosion
door handles, hinges
bronze
copper & tin
hard & strong, and resistant to corrosion
medals, sculptures
stainless steel
iron, chromium, nickel, carbon
corrosion resistant
cutlery, surgical instruments