Youngless: Molecular Geometry
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
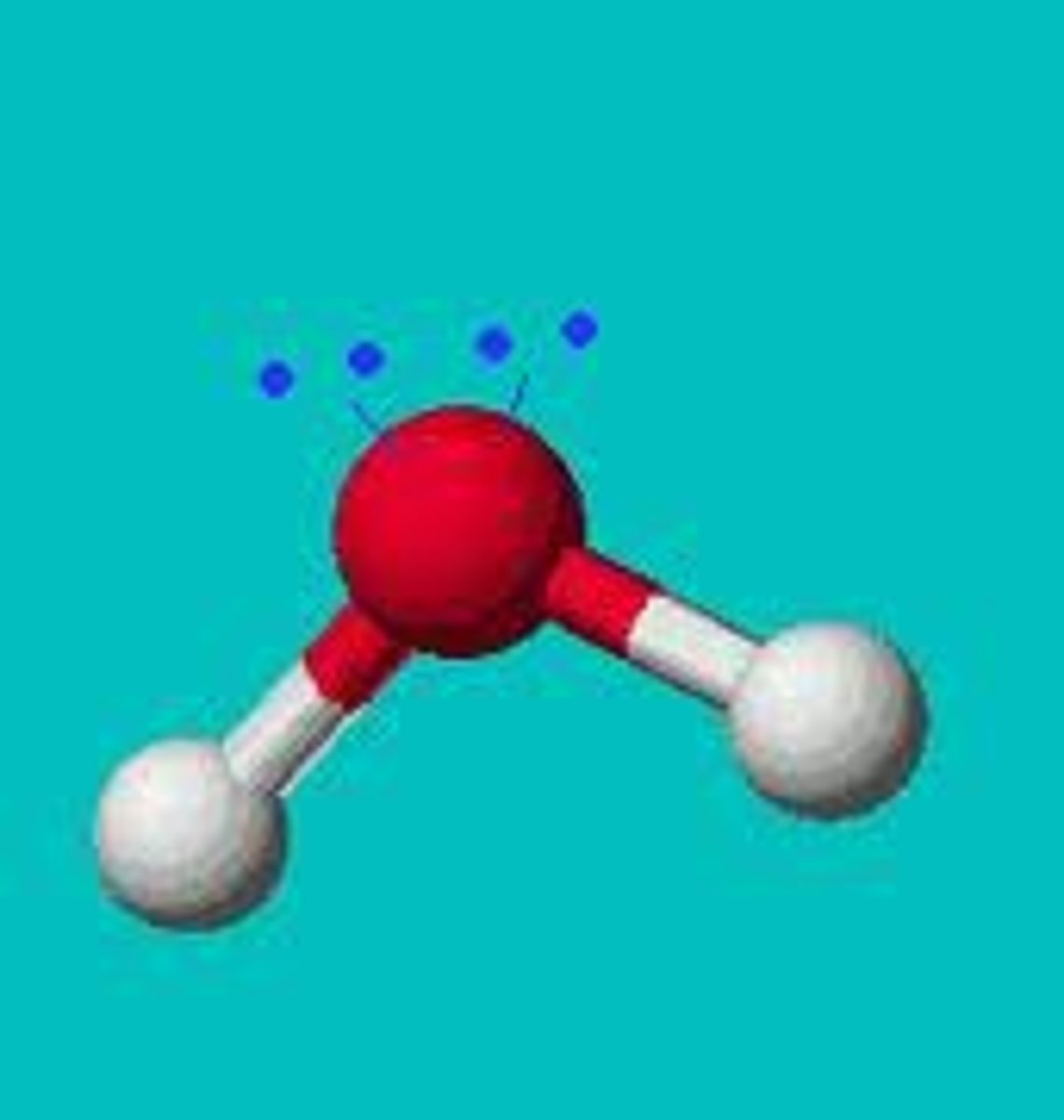
2 Bonds 2 lone pairs
or
2 bonds 1 lone pair
Bent
2
2 bonds 0 lone pairs
Linear

3
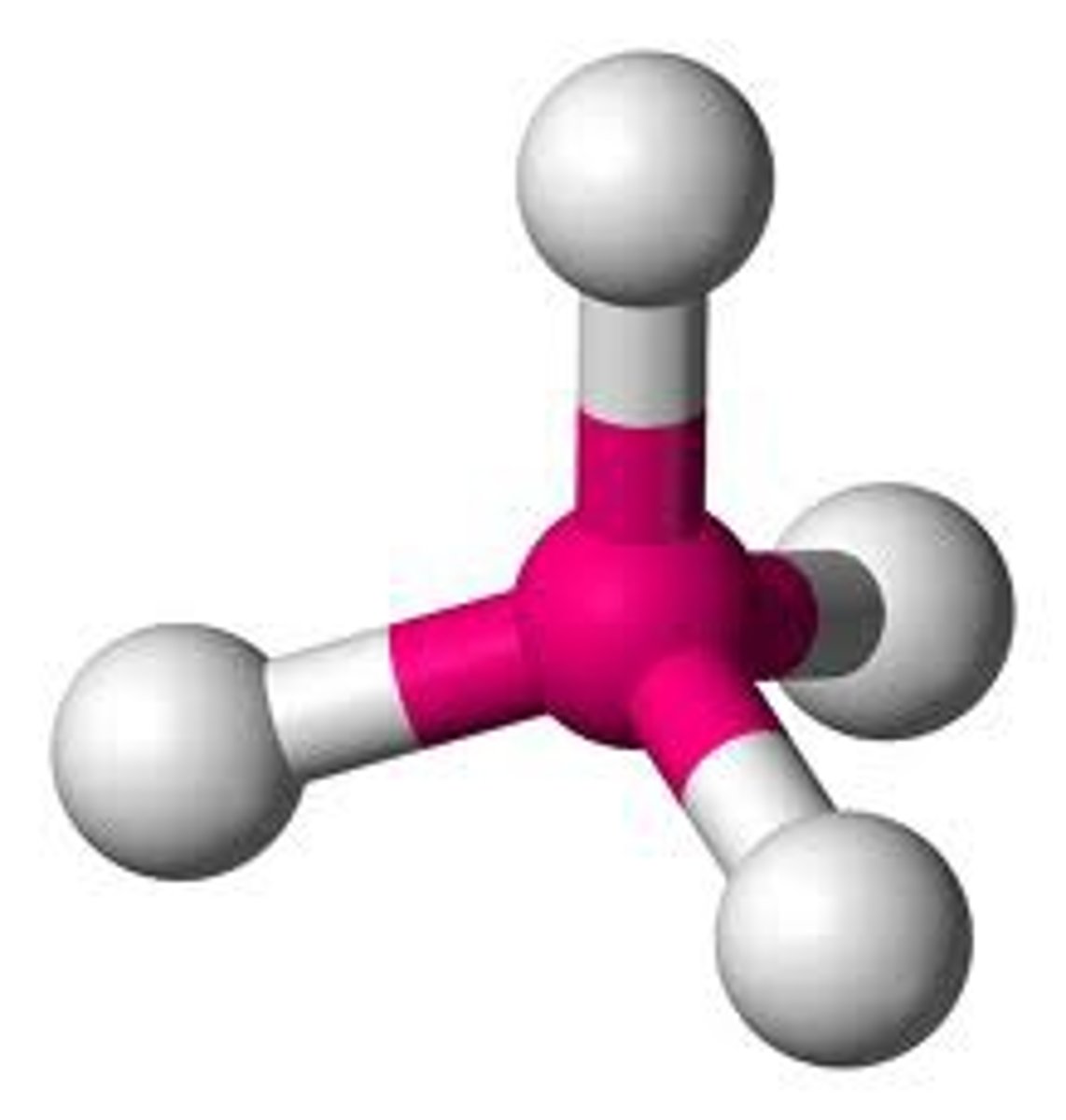
4 bonds 0 lone pairs
Tetrahedral
4
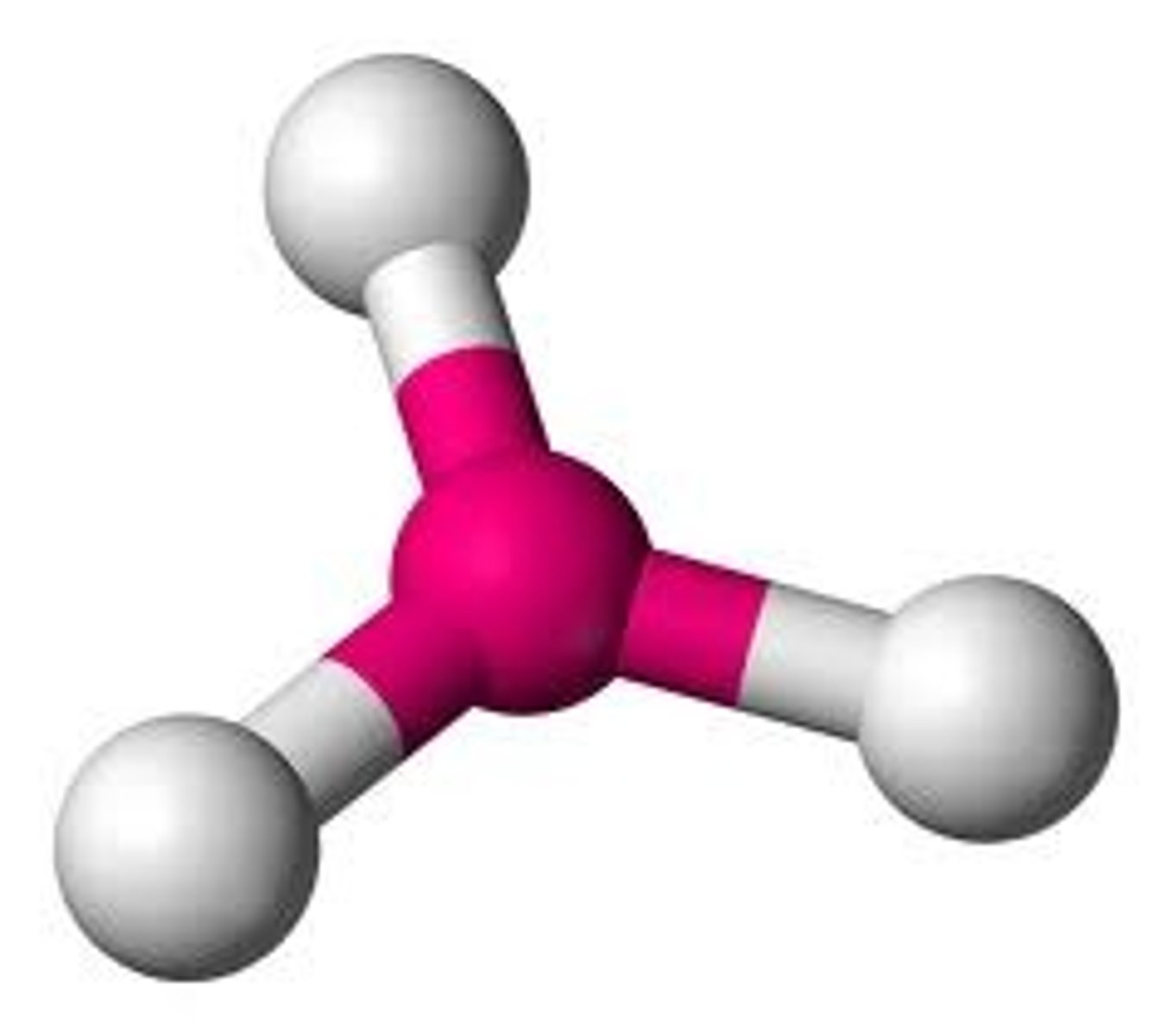
3 bonds 0 lone pairs
Trigonal Planar
5
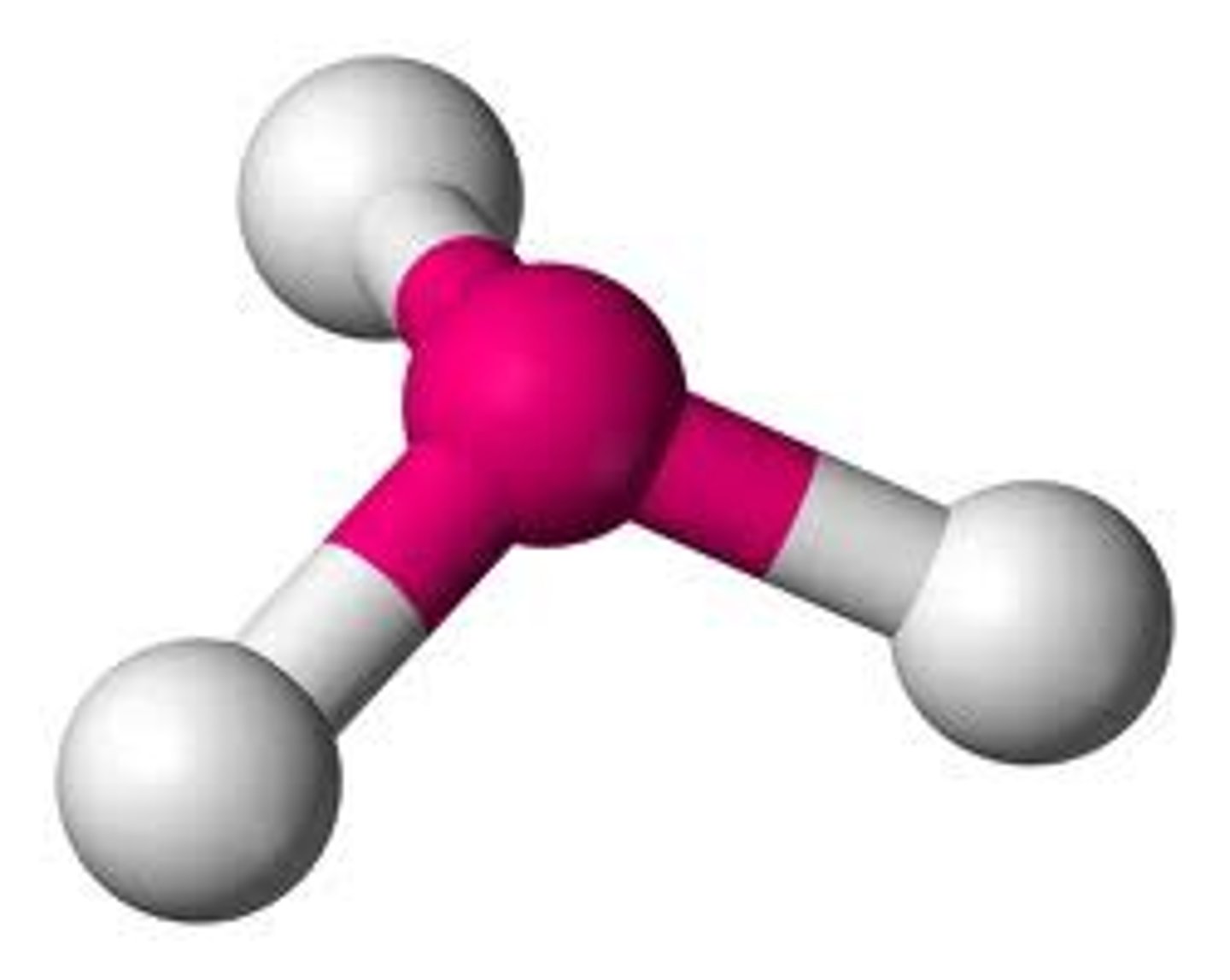
3 bonds 1 lone pair
Trigonal Pyramidal
6
always polar shapes
- Bent
- Trigonal Pyramidal
7
Sometimes Non Polar Shapes
When the same atoms are bonded to central atom
- Tetrahedral
- linear
-trigonal planar
8
Sometimes Polar Shapes
When different Atoms are bonded to central atom
-linear
-trigonal planar
-tetrahedral