BIOL-1050 Practical Exam Study Guide
1/18
Earn XP
Description and Tags
Clemson
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
How do you plot absorbance over time in Excel?
In the first row of the spreadsheet, plot your time (skip the A1 box, start at B1). In column A, input the sample.
Fill out values recorded
Select your entire data by highlighting the relevant cells. Then, go to the "Insert" tab, choose "Chart," and select "Scatter”.
On your graph, double click your points and add a gradient line
Then, right click your points and click “Add Trendline”. On that screen, find the box that says “Display Equation on chart”.
Done! Your result should look like the picture attached.

What is the relationship between pH and pOH?
pH + pOH = 14
How do you find pOH?
14 - pH = pOH
How do you find [H+]?
[H+] = 10^-pH (just plug in the value of pH)
How do you find [OH-]?
[OH-] = 10^-pOH (just plug in the value of pOH)
How do you find pH (with a formula)?
pH = -log[H+] (just plug in the value of [H+])
Percent Solution
Grams required = (% desired) x (volume desired in mL)/100 mL
Percent by volume solution
C1V1 = C2V2 ; C1V1 is the concentration and volume of the stock solution and C2V2 is the concentration of the diluted solution.
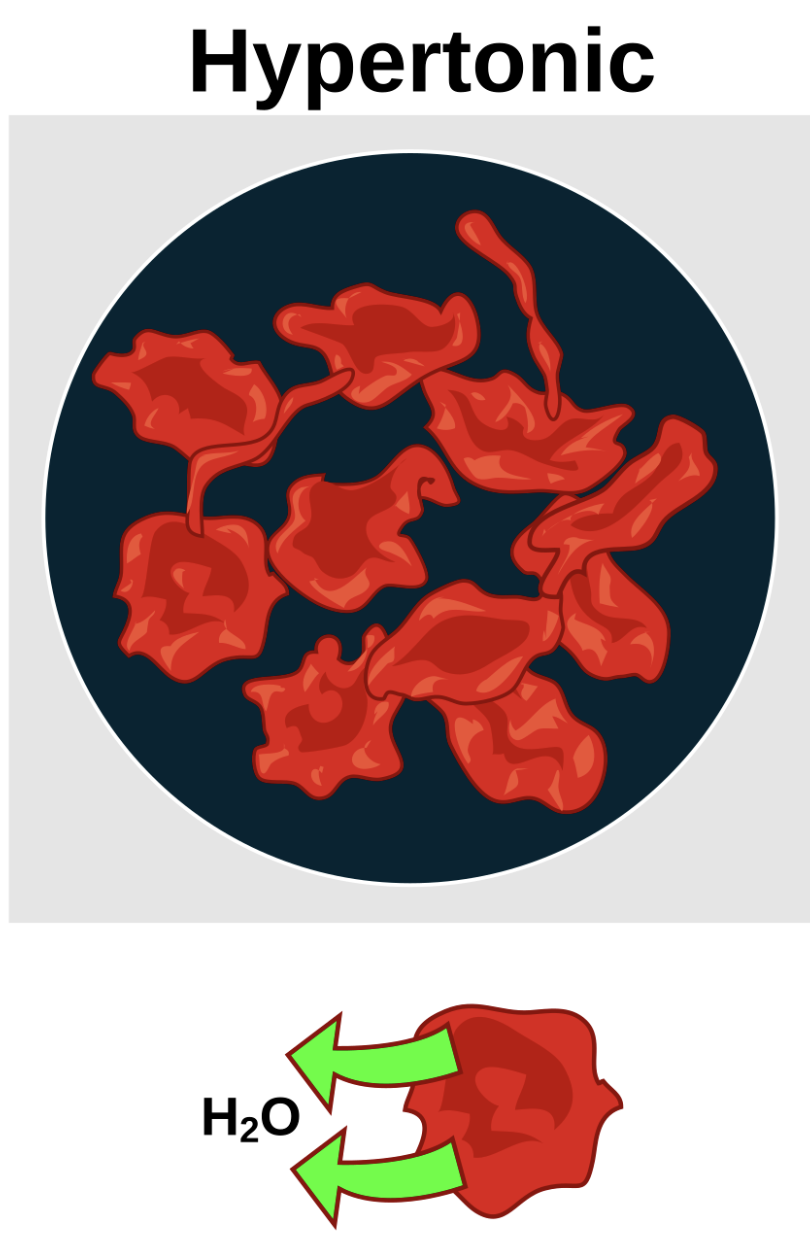
Hypertonic Solution
A solution that has a higher concentration of solutes compared to the inside of a cell, causing water to flow out of the cell, leading to the cell to shrivel.
Hypotonic Solution
A solution that has a lower concentration of solutes compared to the inside of a cell, resulting in water flowing into the cell, which can cause it to swell or burst.

Volume Ratio of a Cylinder
Surface area/Volume
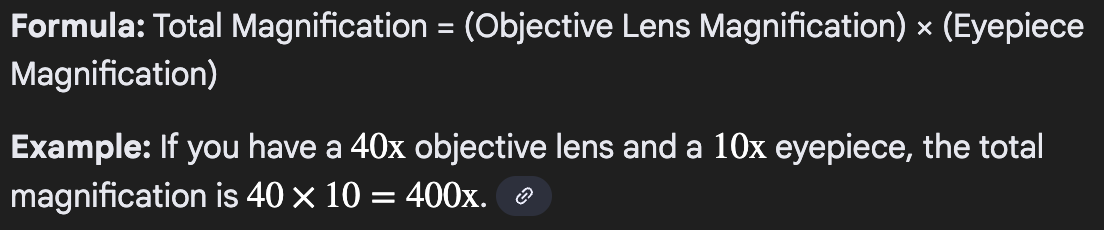
How do you calculate the total magnification of an image?
Multiply the power of the ocular lens by the power of the objective lens used.
Prokaryotic vs Eukaryotic


What kind of cell is this?
Diatoms
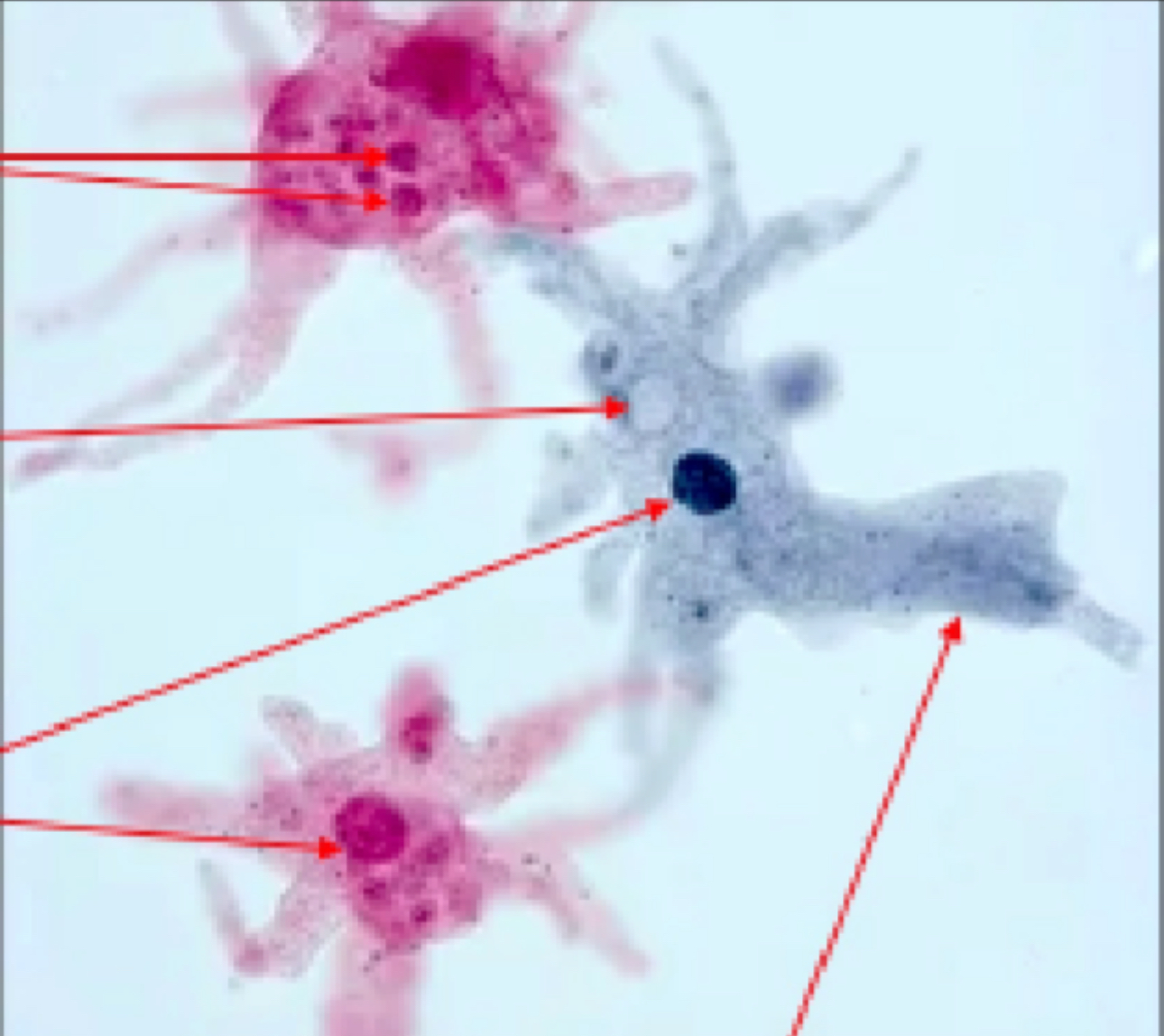
What kind of cell is this?
Amoeba Cells
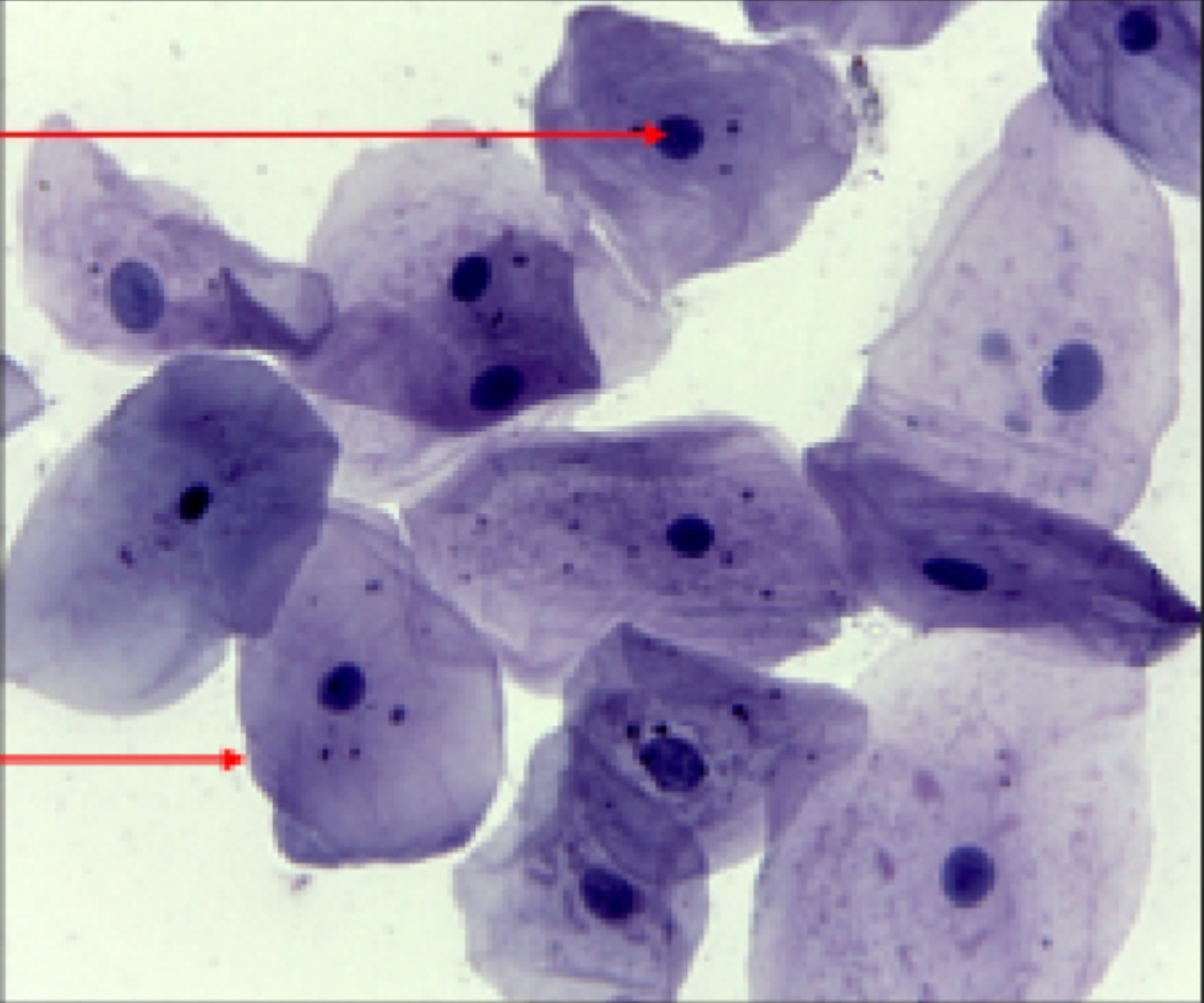
What kind of cell is this?
Cheek Cells

What kind of cell is this?
Elodea Leaf Cells

What kind of cell is this?
Onion Root Cells