CH 221z: VSEPR, bond angles, hybridization, polyatomic ions, and prefixes
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
MG: Linear
Bond angles:180
MG: Trigonal planar
Bond angles:120
EG: Trigonal planar
MG: Bent
Bond angles: <120
MG: Tetrahedral
Bond angles: 109.5
MG: Trigonal pyramidal
Bond angles: <109.5
EG: Tetrahedral
MG: Bent
Bond angles: <109.5
MG: Trigonal bipyramidal
Bond angles: 120, 90
MG: Seesaw
Bond angles: <90, <120
MG: T-shape
Bond angles: <90
MG: Octahedral
Bond angles: 90
MG: Square pyramidal
Bond angles: <90
MG: Square planar
Bond angles: 90
EG: Linear
Hybrid: sp
EG: Trigonal planar
Hybrid: sp2
EG: Tetrahedral
Hybrid: sp3
EG: Trigonal bipyramidal
Hybrid: sp3d
EG: Octahedral
Hybrid: sp3d2
2 bonding groups
Electron Geometry: Linear
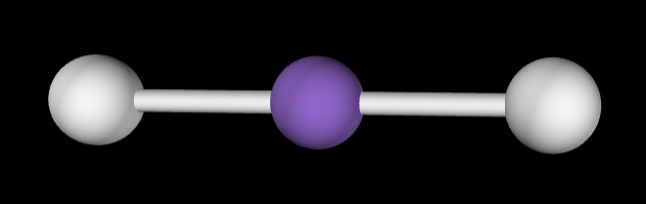
3 bonding groups
Electron Geometry: Trigonal planar
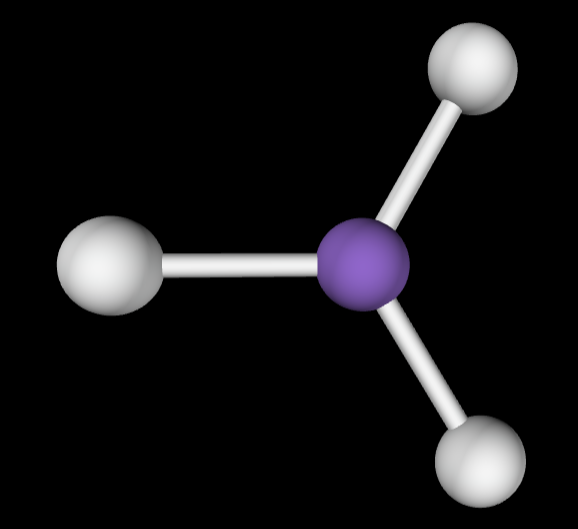
4 bonding groups
Electron Geometry: Tetrahedral
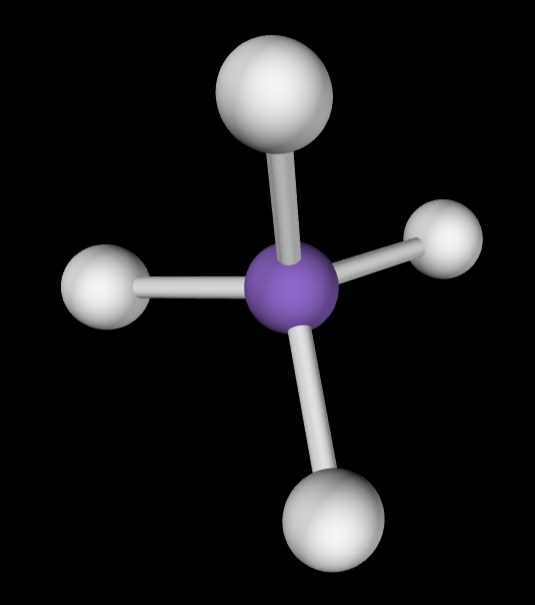
5 bonding groups
Electron Geometry: Trigonal bipyramidal
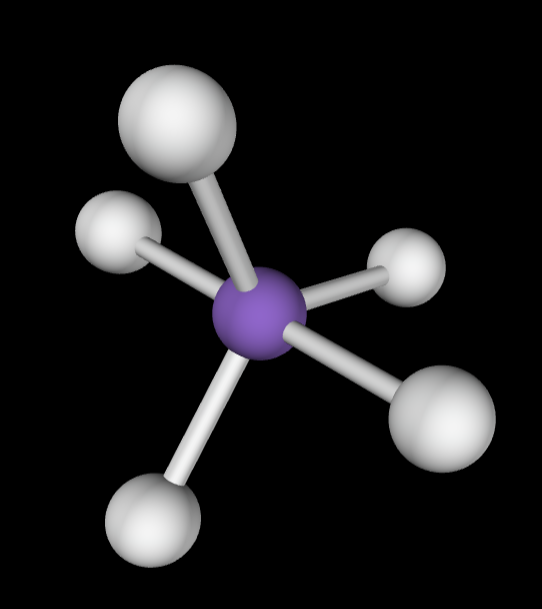
6 bonding groups
Electron Geometry: Octahedral
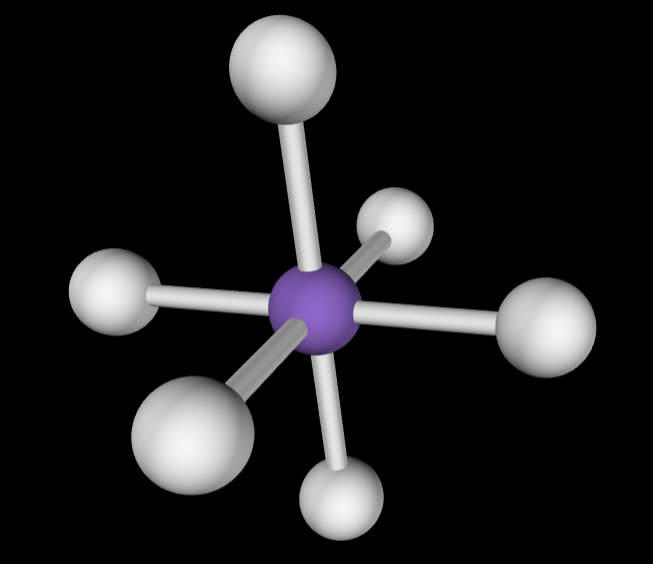
→2 bonding groups
→no lone pairs
Electron Geometry: Linear
Molecular Geometry: Linear
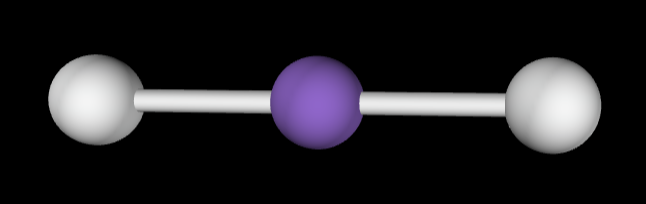
→3 bonding groups
→No lone pairs
Electron Geometry: Trigonal planar
Molecular Geometry: Trigonal planar
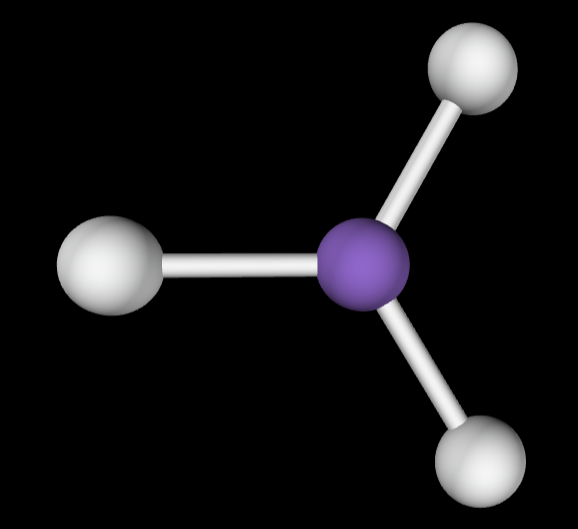
→3 bonding groups
→1 lone pair
Electron Geometry: Trigonal planar
Molecular geometry: Bent
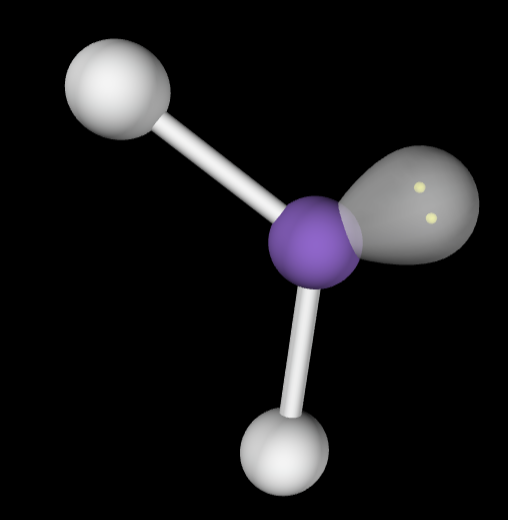
→4 bonding groups
→No lone pairs
Electron Geometry: Tetrahedral
Molecular Geometry: Tetrahedral
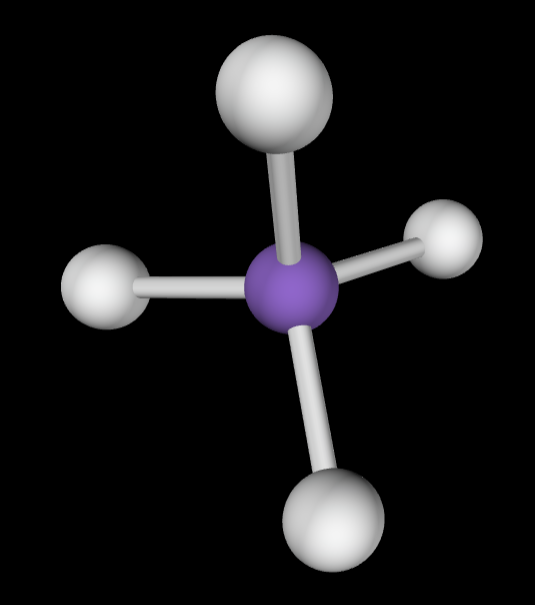
→4 bonding groups
→1 lone pair
Electron Geometry: Tetrahedral
Molecular Geometry: Trigonal bipyramidal
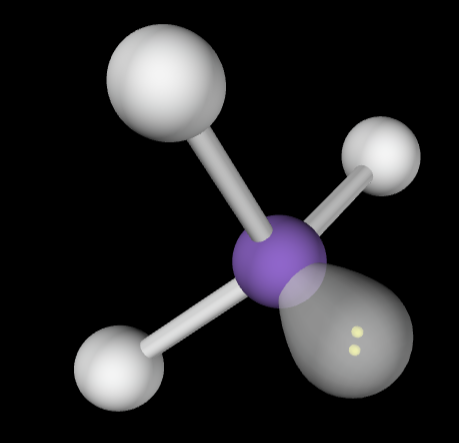
→4 bonding groups
→2 lone pairs
Electron Geometry: Tetrahedral
Molecular geometry: Bent
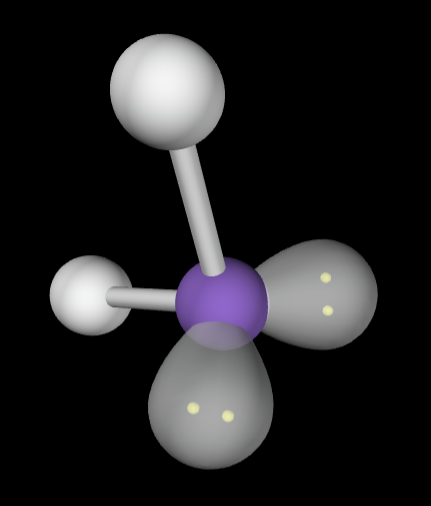
→5 bonding groups
→No lone pairs
Electron Geometry: Trigonal bipyramidal
Molecular Geometry: Trigonal bipyramidal
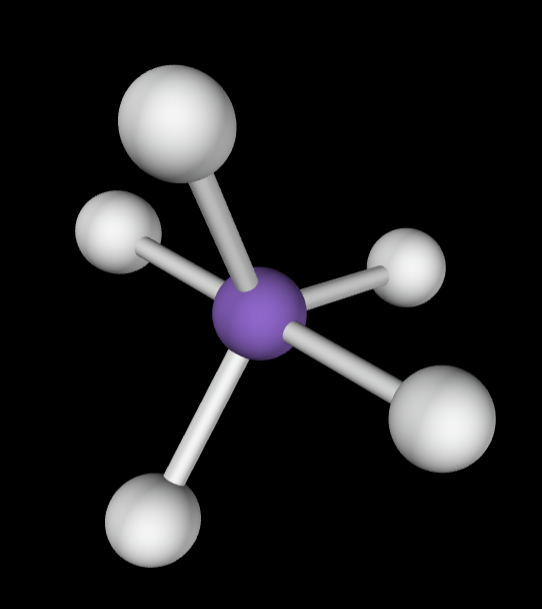
→5 bonding groups
→1 lone pair
Electron Geometry: Trigonal bipyramidal
Molecular Geometry: See-saw
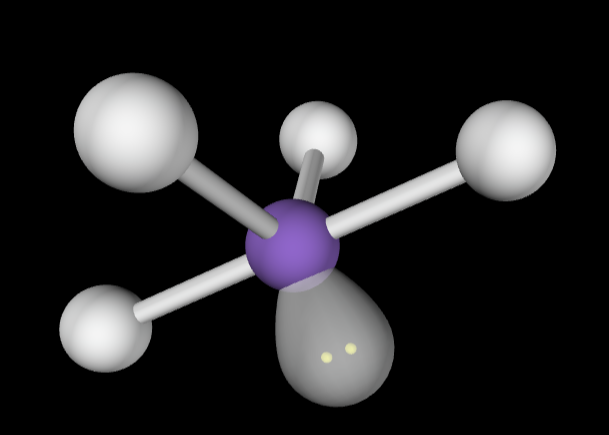
→5 bonding groups
→2 lone pairs
Electron Geometry: Trigonal bipyramidal
Molecular Geometry: T-Shaped
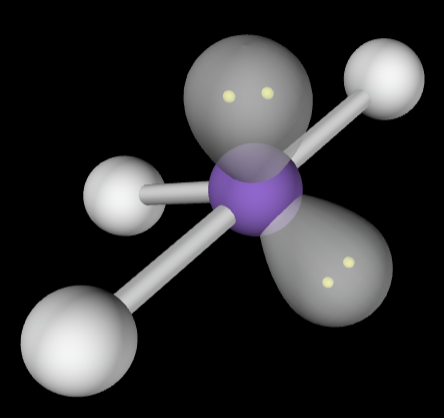
→5 bonding groups
→3 lone pairs
Electron Geometry: Trigonal bipyramidal
Molecular Geometry: Linear
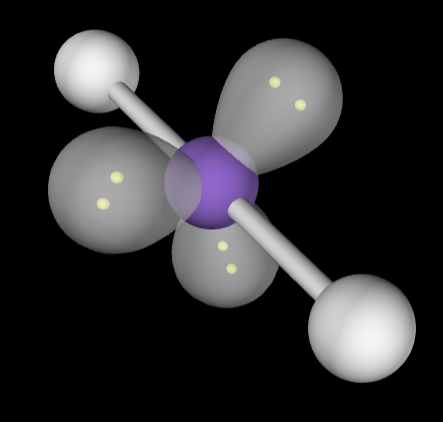
→6 bonding groups
→No lone pairs
Electron Geometry: Octahedral
Molecular Geometry: Octahedral
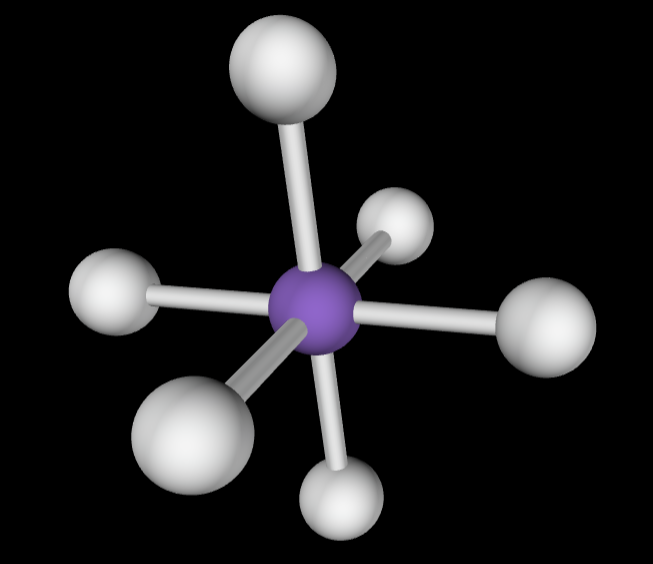
→6 bonding groups
→1 lone pair
Electron Geometry: Octahedral
Molecular Geometry: Square pyramidal
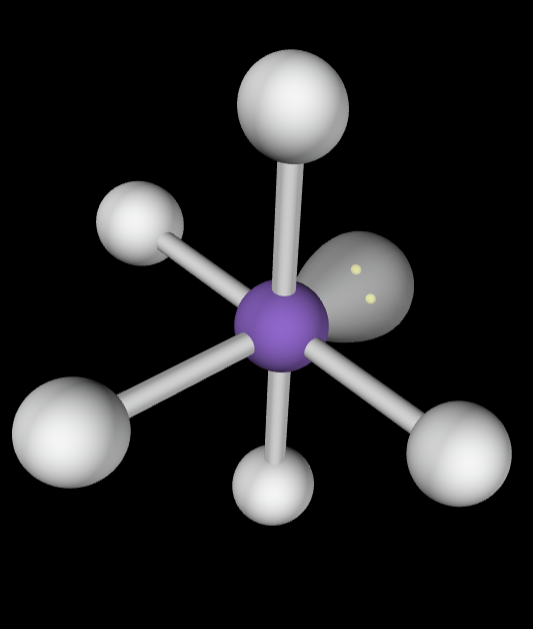
→6 bonding groups
→2 lone pairs
Electron Geometry: Octahedral
Molecular Geometry: Square planar
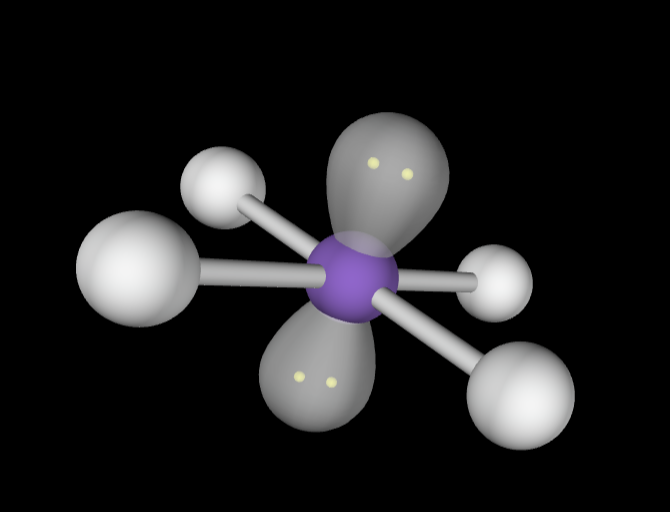
→6 bonding groups
→3 lone pairs
Electron Geometry: Octahedral
Molecular Geometry: T-Shaped
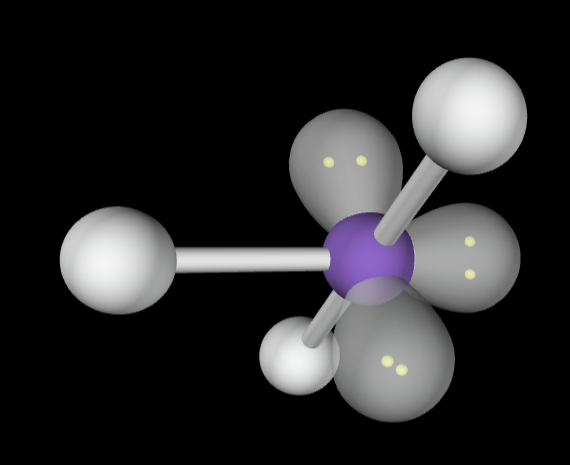
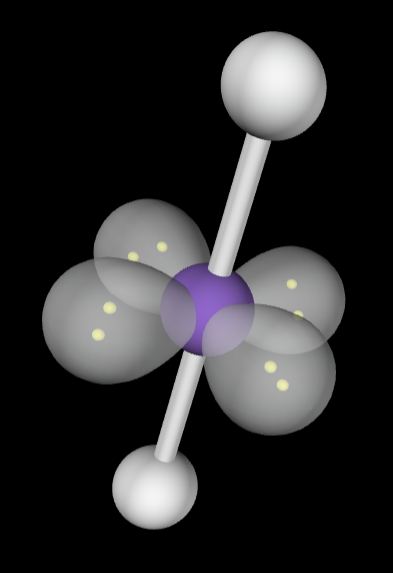
→6 bonding groups
→4 lone pair
Electron Geometry: Octahedral
Molecular Geometry: Linear
Mono-
1
Di-
2
Tri-
3
Tetra-
4
Penta-
5
Hexa-
6
Hepta-
7
Octa-
8
Nona-
9
Deca-
10
Hydroxide
OH-
Cyanide
CN-
Phosphate
PO43-
Nitrate
NO3-
Carbonate
CO32-
Bicarbonate
HCO3-
Sulfate
SO42-
Ammonium
NH4+
Acetate
C2H3O2-