Pharmaceuticals Exam 4
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
94 Terms
How can efficient targeted drug delivery be achieved?
the delivery system must avoid the host’s defense mechanisms (immune system)
circulate to its intended site
taken up by target cells or released in close proximity to target cells
systemic effect
drug acts throughout the entire body
systemic delivery
oral/IV routes
distributed through whole body
local delivery
topical, some injections
enteral for some gastro targets
directly to organ/tissue
local effect
drug affects area in which it was administered
targeted delivery
is generally systemic but drug is forumlated to where it isn’t active everywhere
targeted effect
can occur through tissue/cell-specific delivery
can occur through secondary activation (radiation, heat, light)
can occur through other targeting processes
active targeting
highly specific delivery
precise targeting capability
enhanced delivery efficiency
i.e. organelle targeting, small molecules, antibodies and peptides, aptamer-based, targeting tumor
passive targeting
uses natural physiological processes such as the enhanced permeability and retention (EPR) effect, to gather nanocarriers in diseased tissues (e.g., tumors)
improves bioavailability
selective accumulation
reduces toxic effects
i.e., liposome, polymer, magnetic nano-material, mesoporous silica
target destination
where the drug molecules have to approach to be taken up or exert effects
outside of cell
inside of cell
extracellular structures
target - outside of the cell
changing a signal that is being relayed inside the cell
blocking a signal you dont want transmitted
setting off a signal thats not going off often enough
potential targets- outside of the cell
Any chemical bound to embedded proteins/lipids in cell membrane
Receptors
Ion channels (exterior port)
Channel protein (exterior port)
Membrane-bound enzymes
What are some pre-uptake considerations when it comes to nanoparticles?
• Spherical, larger NPs circulate more easily
• Uncoated/charged NPs quickly cleared by macrophages
• Rod-shaped NPs extravasate more easily
• Rod-shaped, targeted, neutral NPs penetrate tumors best
• Leaky vessels allow larger NPs into tissue
• Positively charged, smaller, coated NPs penetrate mucus more
easily
What is it like to cross the cell membrane as a drug?
Phospholipid bilayer = hydrophobic core (tails) with hydrophilic surfaces (heads)
Challenge: Balance between water solubility
(to dissolve) and lipid solubility (to cross membrane)
• For oral drugs: Must cross intestinal epithelium
• For CNS drugs: Must cross blood-brain barrierKey Principle:
Too hydrophilic → can't cross lipid membrane
Too lipophilic → poor water solubility, can't dissolve/reach membrane
there needs to be a perfect of balance of
properties
Lipinski's Rule of Five (Ro5) - The
Classic Framework
Rule of thumb to evaluate drug-likeness or determine if a chemical compound has
properties that would likely make it an orally active drug in humans
• Formulated by Christopher A. Lipinski in 1997
at Pfizer, based on observation that most
medication drugs are relatively small and
lipophilic molecules
The Four Rules (all multiples of 5)
Poor absorption is more likely when there are:
• LogP (lipophilicity) greater than 5
Rule Interpretation:
• More than 5 hydrogen-bond donors (NH and OH groups)
• More than 10 hydrogen-bond acceptors (N and O atoms)
• Molecular weight greater than 500 Daltons
• A compound can violate ONE of these and still be orally bioavailable
• Violate 2+ rules →possible poor membrane permeability
Understanding LogP - The Lipophilicity Parameter
LogP is directly related to the drug lipophilicity
a key property for the solubility, absorption, membrane
penetration, plasma protein binding, distribution, and tissue penetration
• Partition coefficient (P) measures ability of
compound to differentially dissolve in
mixture of water and lipids/organic solvents
• P = [concentration in organic phase] / [concentration in aqueous phase]
• LogP = log₁₀(P)
LogP Values and Meaning
N-octanol is a fatty alcohol – lipid solution
• Negative LogP = hydrophilic (prefers water)
• Positive LogP = lipophilic (prefers lipids)
• LogP = 0 = equal solubility in both phases
Optimal LogP Values by Drug Type
Oral drugs: LogP <5, ideally between 1.35-1.8 for good oral and intestinal
absorption
• CNS drugs (crossing blood-brain barrier): LogP should be around 2
• Sublingual absorption drugs: LogP should be >5
• LogP value exceeding 5 may exhibit poor absorption or limited membrane
permeability, leading to poor solubility, unpredictable absorption, and
potential accumulation in fatty tissues
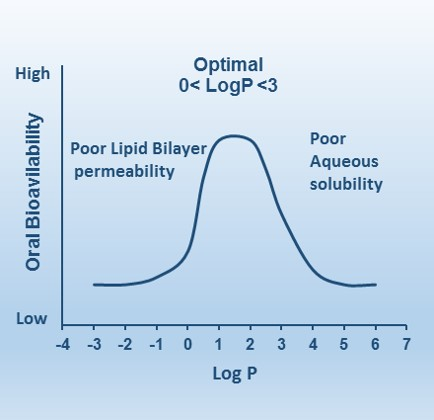
How does the presence of hydrogen bonding affect drug delivery across the cell membrane?
Hydrogen-bond donors (HBD) reduce permeability of compounds into lipophilic
environments
• Hydrogen-bond acceptors (HBA) affect permeability by interacting favorably with strongly hydrogen bonding solvents such as water
• Too many H-bond donors/acceptors → molecule becomes "too comfortable" in water
• High desolvation energy required to leave aqueous environment
• Cannot shed water molecules easily to enter lipid bilayer
• Result: Stuck outside the cell
What is the cellular balance when it comes to hydrogen bonding as a factor in drug delivery?
Hydrogen bond acceptors and donors can form interactions with water,
enhancing solubility, but excessive hydrogen bonding can reduce
permeability by increasing energy required to desolvate the molecule
• Need enough for solubility but not so much that membrane crossing is
impossible
Why is it important to keep the molecular weight lower than 500 Daltons?
Larger molecules have:
• More surface area to desolvate
• Increased molecular complexity
• Greater steric hindrance
• Lower diffusion rates
What is the relationship between molecular size and permeability across the cellular membrane?
Small molecules (MW 150-350 Da) → generally cross membranes well
Medium molecules (MW 350-500 Da) → cross if other properties favorable
Large molecules (MW >500 Da) → increasingly difficult to cross passively
May require active transport or endocytosis
Some successful drugs violate MW rule, often require special transport
mechanismsExamples: Cyclosporine (MW 1203), many antibiotics
What are some compounds that cross membranes easily?
small, lipophilic molecules
molecules that have a small MW 250-350 Daltons (enables better diffusion)
few H-bond donors: 0-2
Few H-bond acceptors: 2-5
uncharged at physiological pH
LogP of 1-3 (balanced lipophilicity)
What are some examples of drugs with great membrane permeability?
Aspirin
• MW = 180.16 g/mol, log P = 1.2, hydrogen bond
acceptors = 4, hydrogen bond donors = 1
• All parameters well within Ro5
Ibuprofen
• MW = 206.29 g/mol, log P = 3.97, hydrogen bond
acceptors = 2, hydrogen bond donors = 1
• Highly lipophilic but still balanced
Caffeine
• Small, lipophilic, crosses BBB easily
• MW ~194 Da, LogP ~0
Steroid Hormones (testosterone, estrogen)
• Very lipophilic
• Cross membranes by passive diffusion
• Can enter nucleus
What are some special cases of membrane permeability?
Uncharged molecules cross membranes much better than charged molecules
Ionized molecules = trapped (can't cross lipid bilayer) (K+, Ca+, etc)
Drug Classes That Exploit This
• Weak Acids (aspirin, ibuprofen)
• Uncharged in acidic stomach (pH 1-3)
• Absorbed in stomach
• Weak Bases (morphine, codeine)
• Uncharged in basic intestine (pH 7-8)
• Absorbed in small intestine
What are some membrane permeability enhancers?
Formulation Strategies:
• Lipid-based formulations (micelles, liposomes)
• Nanoparticles to facilitate endocytosis
• Permeation enhancers (surfactants, bile salts)Chemical Modifications:
• Prodrugs - Add lipophilic groups temporarily
• Cleaved off after absorption
• Example: Ester prodrugs
• Masking polar groups
• Intramolecular H-bonds reduce effective polarityCell-Penetrating Peptides (CPPs):
• Arginine-rich sequences
• TAT peptide from HIV
• Can carry larger, polar cargoDesign substrates for influx transporters
• Amino acid transporters (L-DOPA)
• Glucose transporters
• Peptide transporters (some antibiotics)
Is Ro5 Still Relevant?
Many chemists think of rule of 5 as guardrails
rather than rulesSuccessful Drugs That Break Ro5
Cyclosporine (immunosuppressant) - MW
1203Venetoclax (cancer) - MW 868
Many antibiotics
Macrocyclic drugs
As more molecules that break rule of 5 have
become oral drugs, researchers have begun to
question its valueCan use computational tools for shape/interaction
predictions
What are some ways that drugs can cross cellular barriers?
Uptake through cell membrane
Endocytosis
Exiting vesicular compartments
Crossing additional intracellular
membranes
What are the basic types of membrane transport?
Passive transport- Diffusion
Cross the cell membrane towards the lowest
concentration
Passive transport - Facilitated diffusion
Travel through ion channel
Transport protein which does not use ATP
Active transport
Carrier protein uses ATP to pull drug against the concentration gradient
Endocytosis
Cell “engulfs” ligands which have attached
to receptors on the outside of the cell
What are the four types of endocytosis?
caveolin-mediated endocytosis
clathrin-mediated endocytosis
independent endocytosis
pinocytosis
Pinocytosis
Non-specific uptake of extracellular fluid and dissolved solutes
"Cell drinking" - continuous process in most cells
Forms small vesicles (typically <100
nm)Does NOT require specific receptors
Constitutive process (always occurring at low levels
Mechanism:
Plasma membrane invaginates to form small pockets
Pockets pinch off to form fluid-filled vesicles
Vesicles contain whatever was in the surrounding fluid (non-selective)
Vesicles delivered to early endosomes
How can pinocytosis be used for drug delivery?
Allows uptake of small molecules
and nutrientsCan be exploited for non-targeted
drug deliveryLess efficient than receptor-
mediated pathwaysUseful for drugs that don't have
specific cellular targetsLimited control over uptake amount
receptor-independent endocytosis (macropinocytosis)
Non-specific, large-scale uptake mechanism
Forms large vesicles called
macropinosomes (0.5-5 μm - MUCH larger than other types)Does NOT require specific receptors
Can be enacted on its own OR induced by growth factors/stimuli
causes "Ruffling" of plasma membrane
Macropinocytosis mechanism
How does it work?
Stimulus triggers actin polymerization at cell surface
Membrane extends outward forming lamellipodia or "ruffles"
Ruffles fold back and fuse with plasma membrane
Large vesicle (macropinosome) engulfs
extracellular fluidMacropinosomes mature and can fuse with lysosomes OR recycle back
Triggers/Stimuli:
Growth factors (EGF, PDGF)
Bacterial toxins
Viruses (some hijack this pathway)
Tumor cells often have increased macropinocytosis
What are the advantages of macropinocytosis when it comes to drug delivery?
No receptor required - useful when target cells lack specific receptors
Large cargo capacity - can internalize large nanoparticles, aggregates
High fluid uptake - can deliver more drug molecules
Exploited by some viruses and bacteria (can learn from nature)
What are the drug design considerations when using drug delivery systems that utilize macropinocytosis?
Can be enhanced with cell-penetrating
peptides (CPPs)Useful for large nanoparticles (>200 nm)
that don't fit in clathrin/caveolin vesiclesArginine-rich peptides and proteins can trigger macropinocytosis
What are the cell types that use macropinocytosis?
Cancer cells - particularly RAS-mutated tumors (use for nutrient scavenging
Immune cells (macrophages, dendritic cells) - antigen sampling
Epithelial cells
What are the challenges of macropinocytosis drug delivery?
Less predictable than receptor-mediated pathways
Variable between cell types and conditions
Cargo still may end up in lysosomes
Need to ensure sufficient uptake in target cells
Clathrin-mediated endocytosis
Most common and well-studied form of endocytosis
Receptor-mediated process (highly specific)
Forms clathrin-coated pits → clathrin-coated vesicles
Vesicle size: ~100-150 nm diameter
How does clathrin-mediated endocytosis work?
Cargo binding: Ligand (drug, protein, nanoparticle) binds to
specific cell surface receptorCoat assembly: Receptors cluster in clathrin-coated pits
Vesicle formation: Membrane invaginates, clathrin lattice forms
cage-like structurePinching off: Dynamin (GTPase) pinches vesicle from membrane
Uncoating: Clathrin coat removed, vesicle fuses with early
endosome
What are the advantages of clathrin-mediated endocytosis for drug delivery?
Highly specific targeting - can design ligands for specific
receptorsEfficient uptake - receptor-mediated = high affinity
Well-characterized pathway - predictable intracellular
traffickingNatural pathway for many proteins (transferrin, LDL,
growth factors)
Common Targets/Receptors Used:
Transferrin receptor (iron uptake) - often overexpressed in cancer
LDL receptor (cholesterol uptake)
Folate receptor (overexpressed in many cancers)
EGF receptor (epidermal growth factor)
What are the considerations for drug delivery that utilizes clathrin mediated endocytosis?
conjugate drugs/NPs with ligands for specific receptors
Vesicles traffic to acidic endosomes (pH ~5-6)
Must plan for endosomal escape if cytoplasmic delivery needed
Example: Antibody-drug conjugates often use this pathway
caveolin-mediated endocytosis
Receptor-mediated endocytosis via caveolae ("little caves")
Caveolae = small flask-shaped invaginations (50-80 nm)
Enriched in cholesterol and sphingolipids (lipid rafts)
Less common than clathrin-mediated (~30% of cell types have abundant caveolae)
Often avoids lysosomal degradation* (difference from
clathrin!)
How does caveolin-mediated endocytosis?
Ligand binds to receptor in caveolae
Caveolin-1 protein (main structural component) forms coat
Dynamin pinches off vesicle
Vesicle traffics to caveosomes (NOT early endosomes)
Often bypasses lysosomes → can deliver to ER, Golgi, or
cytoplasm
What are the advantages to caveolin-mediated endocytosis drug delivery?
Avoids lysosomal degradation - major benefit for sensitive cargo (proteins, nucleic acids)
It has less acidic compartments than the clathrin pathway
It can deliver directly to specific organelles
Important for uptake of some toxins, viruses, and signaling molecules
What are some cell types with high caveolae expression?
Endothelial cells (critical for transcytosis across blood vessels)
Adipocytes
Smooth muscle cells
Fibroblasts
What are some design considerations when using drug delivery systems that utilize caveolin-mediated endocytosis?
Target ligands: Albumin, folate, cholera toxin B subunit
Good for pH-sensitive drugs (less acidification)
Useful for delivering cargo that needs to avoid degradation
Can exploit for crossing endothelial barriers (BBB, tumor vessels)
Examples: Albumin-bound paclitaxel (Abraxane®) may use this pathway
What are the challenges to using caveolin-mediated endocytosis drug delivery?
Not all cell types express caveolae
Slower process than clathrin-mediated
Less well-characterized than clathrin pathway
What are some drug targets inside of the cell?
Intracellular receptors
Enzymes
Everything involved with protein synthesis/respiration
Ion channels (interior port)
Channel protein (interior port)
Organelles
Vesicles
Nucleic acids
What are some drug targets outside of the cell?
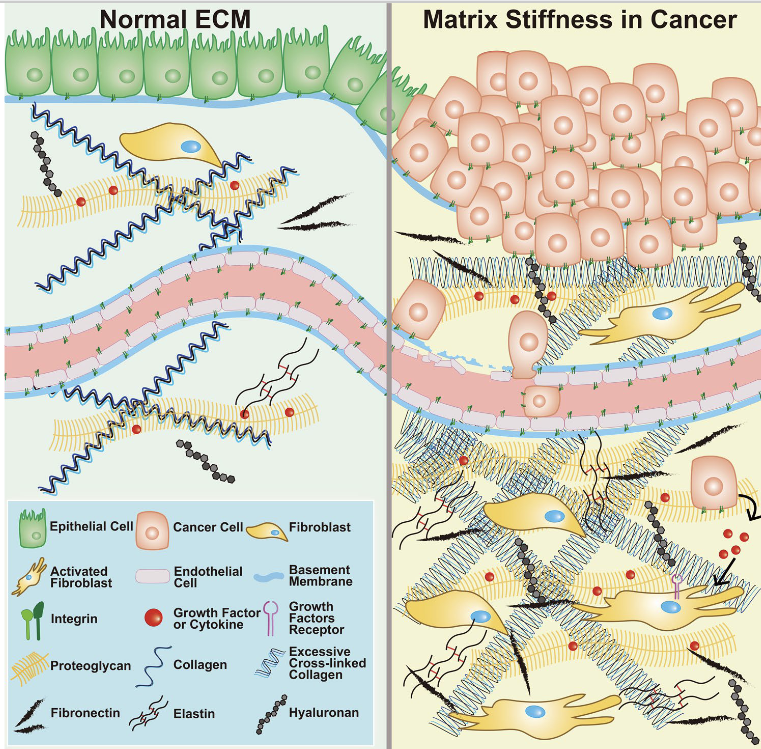
Extracellular matrix (ECM)
Non-cellular, three-dimensional network of macromolecules among cells in tissues
Composed of collagens, elastin, proteoglycans (including hyaluronan), and non-collagenous glycoproteins
Provides structural support AND generates signaling to control cell behavior
How do molecules exit vesicular compartments?
NPs or drugs remain trapped within vesicular compartments, or endosomes, that feature various characteristics such as internal or external receptors.
To achieve functional delivery, most NPs must escape from these compartments before they acidify.
Thus, responsive NPs — such as ionizable NPs that become charged in low- pH environments aid in endosomal escape and allow for intracellular delivery
Unresponsive NPs often remain trapped and are destroyed by lysosome acidity and proteolytic enzymes
Why do drugs target the extracellular matrix?
There is presumably no disease without quantitative and/or qualitative changes in the ECM
In tumors, ECM acts as a barrier shielding cells from therapeutic agents
Dense ECM network causes high interstitial fluid pressure, hypoxia, and reduced effectiveness of chemotherapy, radiotherapy, and immunotherapy
Two Main Strategies:
Direct ECM targeting - Modify/degrade ECM components to improve drug penetration
ECM-based drug delivery - Use ECM molecules or ligands as targeting systems
What are the components in the extracellular matrix that are targeted and present in cancer cells?
Collagen
Most abundant structural protein
Excessive collagen deposition increases ECM stiffness and impairs drug penetration
Target for degradation to improve access
Hyaluronic Acid (HA)
Highly expressed in tumor extracellular matrix
Creates dense, hydrated barrier
Can be targeted by hyaluronidase enzymes
Fibronectin Cell
adhesion protein
Promotes tumor invasion and metastasis
Integrins (ECM receptors)
Transmembrane receptors whose primary role is to recognize and bind
Target for drug delivery via ECM ligands
Strategy 1 to combat ECM barrier for drug targeting - Use enzymes or other agents to degrade ECM and improve drug penetration
Overcome physical barrier to drug delivery
Reduce interstitial fluid pressure
Improve oxygenation (better radiation therapy response)
Hyaluronidase (HAase) - Targeting Hyaluronic Acid:
Nanoparticles modified with hyaluronidase on the surface can degrade hyaluronic acid in tumor ECM
Allows deeper penetration of chemotherapy drugs
Collagenase:
Degrades collagen fibers
Reduces tissue stiffness
Improves drug distribution
Sequential Drug Release System
Strategy 2 to combat ECM barrier for drug targeting - Use ECM-binding ligands for targeting
Targeting Through ECM Receptors:
Integrin Targeting (αvβ3, αvβ5)
RGD peptides (Arg-Gly-Asp) bind to integrins
c(RGDyK) peptide targets integrin αvβ3 expressed on tumor cells
Hyaluronic Acid Receptor Targeting (CD44)
CD44 overexpressed on many cancer cells
HA-conjugated drugs bind to CD44
Triggers receptor-mediated endocytosis
Collagen Receptor Targeting
Discoidin domain receptors (DDRs)
Integrins that bind collagen
Strategy 3 to combat ECM barrier for drug targeting - Tumor Penetrating Peptides (TPPs)
Peptides that bind to ECM components and trigger trans-tissue transport
Help drugs penetrate through dense tumor stroma
Key Example - iRGD Peptide:
Mechanism:
Binds to αv integrins on tumor endothelium
Proteolytically cleaved in tumor
Exposed CendR binds neuropilin-1 receptor
Triggers transcytosis through tissue
Enhances drug penetration into tumor core
What are some current drugs that act on the extracellular matrix indirectly?
Angiotensin Receptor Blockers (ARBs):
Examples: Losartan, valsartan
Block angiotensin II type 1 receptor, showing antifibrotic action by reducing ECM
accumulation
Clinical use: Significantly slow the rate of progressive aortic-root dilation in Marfan's syndrome patients
TGF-β Inhibitors:
Target key pro-fibrotic signaling
Prevent excessive ECM deposition
Calcium Channel Blockers:
Examples: Nifedipine, amlodipine
Act on ECM via suppressing TGF-β, resulting in reduced matrix accumulation
Key Point: These drugs weren't designed to target ECM, but ECM modulation contributes to their therapeutic benefit
What are the advantages of extracellular matrix-targeted therapy?
Universal tumor targeting
Potential to affect all tumor cell types, including tumor stem cells and cancer-associated fibroblasts
Not limited to cells expressing specific markers
Overcome drug resistance
ECM doesn't mutate like cancer cells
More stable target
improve penetration
Breaking down ECM allows better drug distribution
Enhances effectiveness of other therapies
Reduce side effects
ECM-drug conjugates showed significant reduction in doxorubicin-induced cardiotoxicity
More targeted delivery = less systemic toxicity
What are the challenges of extracellular matrix-targeted therapy?
• Must distinguish tumor ECM from normal tissue ECM
• Risk of affecting healthy connective tissue
• ECM composition varies between tumor types
• Multiple components need simultaneous targeting
• Getting large enzymes (like hyaluronidase) into tumors is hard
• Ensuring sustained activity at tumor site is also hard
• ECM degradation might facilitate tumor cell escape (which is really bad)
antibody drug conjugate
A type of biopharmaceutical that enables the targeted delivery of cytotoxic agents to tumor sites by recognizing target antigens by antibody while minimizing dmage to healthy tissues
Components:
the antibody part
the cytotoxic chemo/payload/warhead - binds to intracellular target to disrupt cellular process to promote cell death
the linker protein that connects the antibody and chemo
Example:
Trastuzumab emtansine (Kadcyla®): This ADC treats metastatic HER2-positive breast cancer.
What are some targeted drug delivery methods that utilizes antibodies?
Antibody-Drug Conjugates (ADCs)
Targeted therapy and
chemotherapyTraditional chemotherapy is
systemic
red blood cell
• Erythrocyte – erytho = red, ctye =
cell
• Largest population of cells
• No nucleus
• Packed with hemoglobin
• Biconcave shape allows for passage
through narrow capillaries
• Live in bloodstream for 100-120
days
What are some drug loading methods for red blood cells?
Drugs attached to surface
Drugs incorporated into cell
Multiple methods
Electric pulse/ultrasound – disrupt cell membrane to let in
particlesEndocytosis – cell engulfs a drug-containing particle
Osmotic – change the water balance of the cell to push or pull drug inside
What are some benefits to red blood cell drug delivery?
Ease of cell isolation in large quantities and the ability to scale production
Biocompatibility
autologous and donor erythrocytes are used to treat patients
Biodegradability
old or damaged erythrocytes
removed by spleen
Long life in the bloodstream
RBCs protect drug from the immune
system and plasma proteasesCells survive in the body for a long time
Pharmacokinetics and pharmacodynamics of the drug in RBCs can significantly increase the desired therapeutic effect
Decreasing side effects of drugs
preventing allergic reactions
the decrease in the peak concentrations of free drug in the blood to safer levels
What were some advancements made in the 2010s?
HIV
• Antiretroviral Dovato 1-2 pills a day vs 4-6
• Fostemsavir – for multi-drug resistant HIV infection
• PrEP (pre-exposure prophylaxis) to prevent transmission
• HPV vaccine approved to prevent HPV infections and cervical
cancer
• First chimeric antigen receptor (CAR) T-cell therapies were
approved by the FDA
• First gene therapy to receive U.S. approval, Luxturna (Spark Therapeutics) 2017 for a rare, inherited eye disease that causes
blindness
• Approval of Zolgensma (Novartis) in 2019 for spinal muscular
atrophy, a deadly muscle-wasting disease which cost $2.1
million
• CRISPR- based medicines launch
Which US war prompted an outcry from the public on how many soldiers died due to adulterated drugs, and led to the passage of the first federal drug law?
Mexican-American War
Mexican-American War (1846-1848)
• During the course of the war, 1,773 Americans were killed in action
with an additional 13,271 dying from other causes.
• This high number of collateral casualties shocked the nation, and
calls came from across America for an investigation —> pharmaceuticals/drugs
What was the conclusion after the investigation of miscellaneous deaths in the Mexican-American War?
It was concluded that adulterated drugs supplied to the Army
had caused the large numbers of deaths among soldiers.
Drug Importation Act (1848)
The outcry led Congress to pass the Drug Importation Act of 1848,
the first federal drug law.
It was very limited in scope and addressed only
the purity of drugs imported into the United States.Congress charged Customs with enforcing the law.
The publication of what book helped fuel the support for the Food and Drug Act of 1906?
The Jungle - Upton Sinclair
Sherley Amendment of 1912
coupled the Amendment of Food and Drug Act of 1906
Regulates manufacturers’ claims of benefits
Prohibits false claims of therapeutic effect
Made it illegal to sell drugs the manufacturer knew to be worthless
What happened during the investigation of sulfanilamide?
Harold Watkins, the chief pharmacist, committed suicide whilst awaiting trial.
Defense: there were no official standards for toxicity testing for them to violate
Company only had to pay fine for calling it an “elixir” when it did not contain alcohol
S. E. Massengill Company
=> Beecham Group
=> SmithKline Beecham
=> GlaxoSmithKline
Diethylene Glycol
A very potent solvent
Useful for many industrial applications
When ingested or applied topically destroys:
Liver
Kidneys
Nerves
Effects were mostly unknown at the time
> 240 gal were distributed
105 patients died
What happened after the investigation of diethylene glycol (DEG)?
Nothing.
Continued to be used in drug manufacturing
Many mass poisonings from contaminated drug
Federal Food, Drug, and Cosmetic Act of 1938
There was a huge public outcry over sulfanilamide
tragedy
Prohibits distribution and use of any new drug or drug product without the filing of a New Drug Application (NDA) and approval of FDA
Established the FDA - U.S. Food and Drug Administration
What does the US Food and Drug Administration do?
It enforced and currently enforces the 1938 act
Grant or denies permission to manufacture/distribute new products
Reviews applicant’s data when considering drug manufacturating or distribution including:
Ingredients
Method of assay
Formulation and manufacturing processes
Preclinical (animal, toxicology) and clinical (human) trials
Required safety for human use
BUT it does NOT address efficacy – whether it works or not because that part is more subjective
Durham-Humphery Amendment of 1951
Amendment to Federal Food, Drug, and Cosmetic Act
Legal distinction between prescription and over-the-counter (OTC) drug
Prior to this, all drugs were classified as OTC
ALL prescription drugs must be labeled
RX Only
“Caution: Federal Law Prohibits Dispensing
Without Prescription”Refills only with consent of provider
Thalidomide
Developed in the 1950s as a sedative/tranquilizer
Used for cold, flu, nausea, morning sickness during pregnancy
No lethal dose found while doing
animal testing – “harmless to humans”Licensed for OTC use in 1956 in Germany
World-wide production - 14 pharmaceutical companies were marketing thalidomide in 46 countries under at least 37 different trade names.
Who was Paracelsus? (ca. 1493-1541 CE)
Aureolus Theophrastus Bombastus von Hohenheim
Swiss physician and chemist
Away from botanical remedies, toward chemistry
Specific medicinal ingredient to combat a specific
disease
Importance of cleanliness, protection of wounds
Toxicology
Psychosomatics – mental wellbeing => physical health
also made some prophecies about the future
What were some historical advances of medicine by the Romans?
Surgical instruments
Forceps
Scalpels
Cross-bladed scissors
Surgical needle
Speculas
Who was Hippocrates? (ca. 460-733 BCE)
Hippocratic Oath – confidentiality and “do no harm”
Illness as an imbalance of internal factors
Not external spirits or supernatural influences
Descriptions of diseases and conditions
Delineation of acute, chronic, endemic, epidemic
Described 100s of drugs
Pharmakon (Gr.) – remedy used for good only
What was ancient medicine?
Early written texts on diagnosing and treating illness in many cultures
Mesopotamia (Bablyon, neo-Assyrian)
Indian
Atharvaveda – charms, spells, herbal remedies
Ayurveda – “complete knowledge for long life”, medical system
Chinese
Traditional Chinese Medicine – herbal medicine, acupuncture, massage
Pharmakon (Gr.) – charm or drug used for good or evil
The Great American Fraud
Gullible America will spend this year some 75 millions
of dollars in the purchase of patent medicines,” one of
Adams’ stories reported. “In consideration of this sum it
will swallow huge quantities of alcohol, an appalling
amount of opiates and narcotics, a wide assortment of
varied drugs ranging from powerful and dangerous
heart depressants to insidious liver stimulants; and far
in excess of all other ingredients, undiluted fraud. For
fraud, exploited by the skillfulest of advertising bunco
men, is the basis of the trade.”
• $2.6 billion in 2024
discusses how American medicine is fueled by dangerous and poisonous substances that keep you within the cycle of fraudulent healthcare, despite the large amount of money that is spent patenting and purchasing medicine. Wealthy corporations that advertise good health are also the ones that control the cycle that keep you sick and needy.
Drug Listing Act of 1972
• Compile a full list of marketed drugs
• National Drug Code
• One unique identifier per drug per package
type per manufacturer
• Permanently assigned
• Manufacturers and re-packagers must register
and supply information about their drug
• NDC must appear on all drug labeling
• Search online via Drug Code Directory
Poison Prevention Packaging Act (PPPA) - 1970
This law was passed to require a number of household substances to be packaged in child-resistant packaging.
According this Act, the packaging must be designed or constructed
to be significantly difficult for children under five years old to open within a reasonable time, and not difficult for normal adults to use properly.
FDA Black Box warning 1979
• Most serious type of warning mandated by FDA
• Prominently featured in the labeling of drugs to warn prescribers about:
Serious adverse reactions, compared to potential benefit, that they should be considering when prescribing
The risk can be prevented/reduced by careful use, FDA has restrictions on when drug can be used
Kefauver-Harris Amendments of 1962
• Amendment to Federal Food, Drug, and Cosmetic Act
• Passed without dissent
• Before clinical trials, manufacturers have to prove new drug is SAFE AND EFFECTIVE
• Investigational New Drug Application (IND) filed and approved
• Perform clinical trials on humans
• Then file an NDA for approval to market
Controlled Substances Act of 1970
• Combined existing federal drug laws and expanded their scope, but it also
• Changed the nature of federal drug law policies
• Expanded federal law enforcement pertaining to controlled substances
• Established new regulatory framework
• Drug Enforcement Administration (DEA) in Dept of Justice
• Established drug Schedules I-V
NY Herbal Supplement Investigation - 2016
The investigation focused on a variety of herbal supplements from four major retailers:
GNC, Target, Walmart and Walgreen Co.
Lab tests determined that only 21 percent of the products actually had DNA from the plants advertised on the labels.
The investigation found supplements, including echinacea, ginseng, St. John's wort, garlic, ginkgo biloba and saw palmetto, were contaminated with substances including rice, beans, pine, citrus, asparagus, primrose, wheat, houseplant and wild carrot.
In many cases, unlisted contaminants were the only plant
material found in the product samples.
Food and Drug Modernization Act of 1997
Streamlined policies and new regulations
Expanded access to investigational treatments for serious illnesses
AIDS, Alzheimers, cancer
Use drug user fees to hire more reviewers, use external reviewers
Incentives for researching drugs for children
Joint program with NIH to track clinical data
Late 2000s in the industry
The average cost and time to develop a new drug continued to increase, often exceeding $1.3 billion and 12 years for a successful launch
• High-profile lawsuits and product withdrawals (such as Merck's Vioxx) led to greater public and regulatory scrutiny of drug safety.
• Pharmaceutical companies increasingly engaged in collaborative partnerships with academic institutions, smaller Biotech firms, and private foundations
• This "open innovation" model aimed to leverage external expertise and share the financial burden of early-stage research
• increased research focus on complex diseases, particularly central nervous system disorders and cancer (antineoplastic agents)
What were some key developments in medicine of the early 2000s?
At Oregon Health and Science University, Shoukhrat Mitalipov and his team cloned a Rhesus Monkey and used the resulting embryo to create stem cells (2007) using somatic cell nuclear transfer (SCNT)
Human Genome Project
Launched in October 1990 and completed in April 2003
Generated the first sequence of the human genome
Affordable Care Act
• Signed in 2010
• Brought healthcare coverage to ~ 20 million uninsured people
• Provided protections for ~ 50 million people who could otherwise have a hard time getting health coverage, thanks to past injuries or illnesses
• No annual or lifetime limits on coverage
• Pregnancy cannot be a “pre-existing condition” and denied coverage
• Center for Medicare and Medicaid Innovation focused on value-based care, or paying doctors and hospitals for making patients healthy, rather than for each visit or surgery
• Supreme Court ruled in 2012 that states could decide whether to expand Medicaid
• Fourteen states haven't, resulting in about 4.9 million more people in the US going without health insurance