B1 Cell-level systems
1/73
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
mm to µm
1mm = 1000µm
Eukaryotic cells
contain genetic material in a nucleus
complex and relatively large
sizes between 10-100µm
plant & animal cells
Prokaryotic cells
do not contain a nucleus, genetic material floats in cytoplasm
simple and relatively small
sizes between 1-10µm
bacterial cells
Differences between plant and animal cells
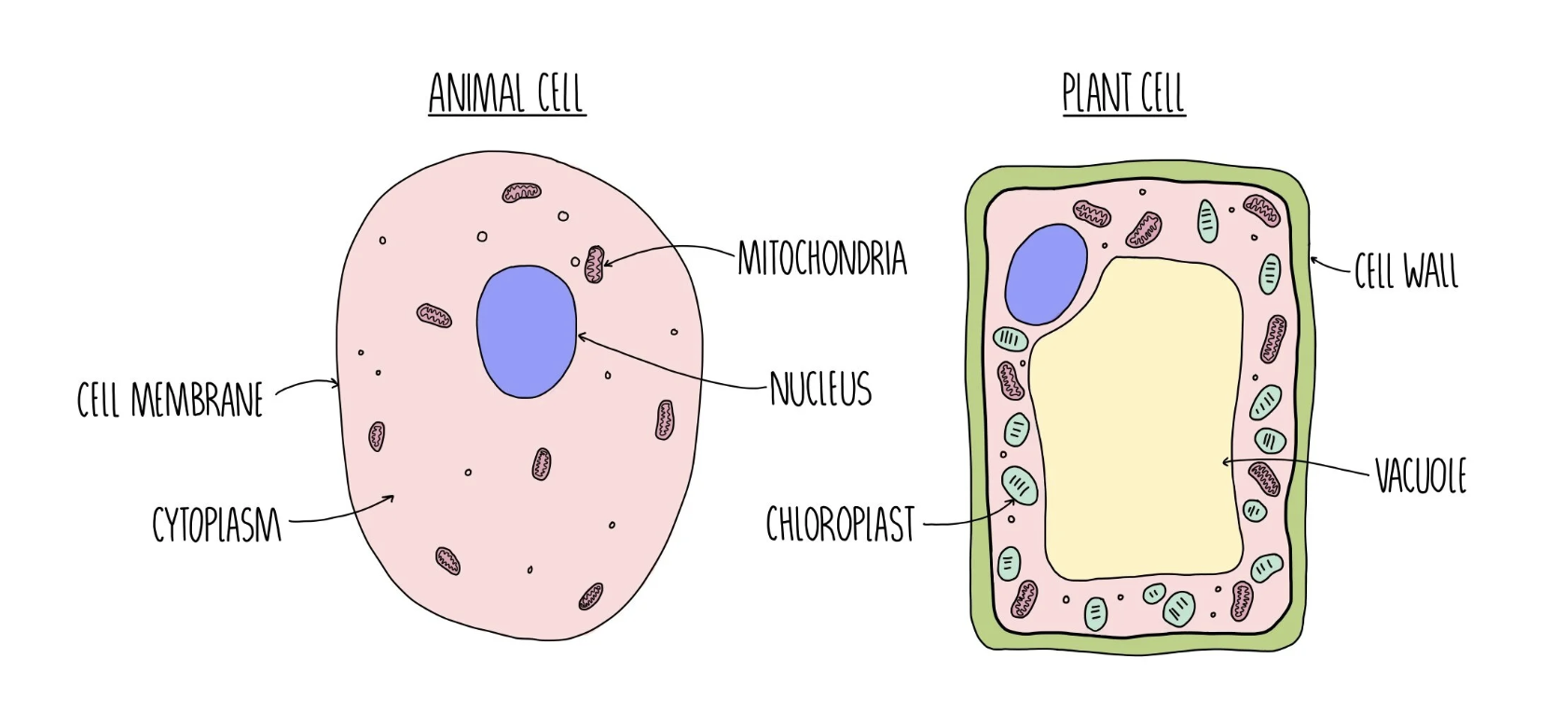
Nucleus
controls the activities of the cell, and contains the organism’s genetic material (eukaryotic cells).
Cell membrane
a selective barrier that controls the movement of substances into and out of the cell.
Cytoplasm
a ‘jellylike’ substance; the chemical reactions that keep the cell alive occur here.
Mitochondria
where respiration happens; enzymes enable glucose and oxygen to react together to release and transfer energy in the form of ATP.
Cell wall
made of a fibre called cellulose (which makes the wall rigid and supports the cell).
Vacuole
full of cell sap, which also helps to keep the cell rigid and supports it.
Chloroplasts
contain chlorophyll, which transfer energy from the Sun to the plant, which is used in photosynthesis to make glucose. (eukaryotic)
Flagella
thin ‘tail-like’ structures that allow the cell to move through liquids. (prokaryotic)
Pili
tiny ‘hair-like’ structures that allow cells to attach to structures. Also used to transfer genetic material between bacteria. (prokaryotic)
Slime wall
protects cell from drying out and from poisonous substances. Also helps bacteria stick to smooth surfaces. (prokaryotic)
Plasmids
circular piece of DNA used to store extra genes. This where antibiotic resistance genes are normally found. (prokaryotic)
Process of applying stain to a colourless cell
place cells on a glass slide
add one drop of the stain
place a coverslip on top
tap the coverslip gently with a pencil to remove bubbles
Resolution
the smallest distance between two points that can be seen as separate entities.
Differences between light & electron microscopes

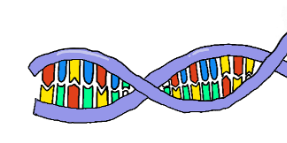
DNA
deoxyribonucleic acid - the molecule that contains genetic material in all organisms. It is a polymer of nucleotides.
Chromosome
a long molecule of DNA; all cells in the human body have 46 chromosomes (diploid) apart from gametes, which have 23 (haploid).
Gene
a small section of DNA on a chromosome; each gene codes for a specific sequence of amino acids to make a specific protein.
Genome
the entire genetic material of an organism.
The shape of DNA
double-helix structure/shape.
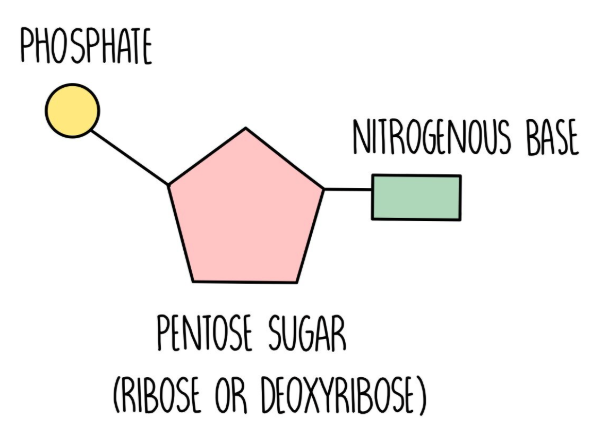
Composition of a DNA nucleotide
phosphate + deoxyribose + base (A,T,C,G)
The rule for base pairing
Complementary base pairing:
A — T
C — G
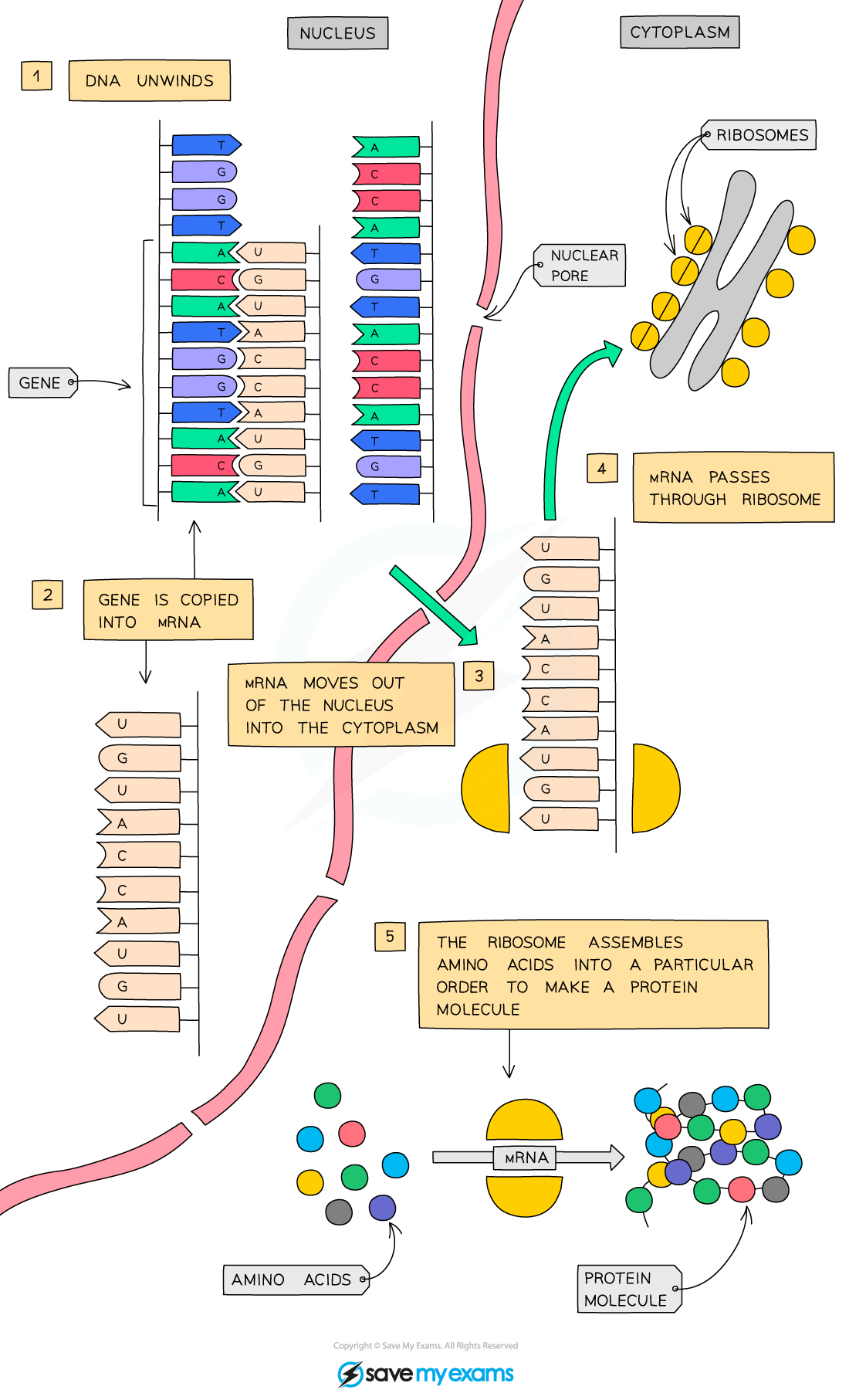
Transcription
the process by which a section of DNA (one gene at a time) is copied onto mRNA.
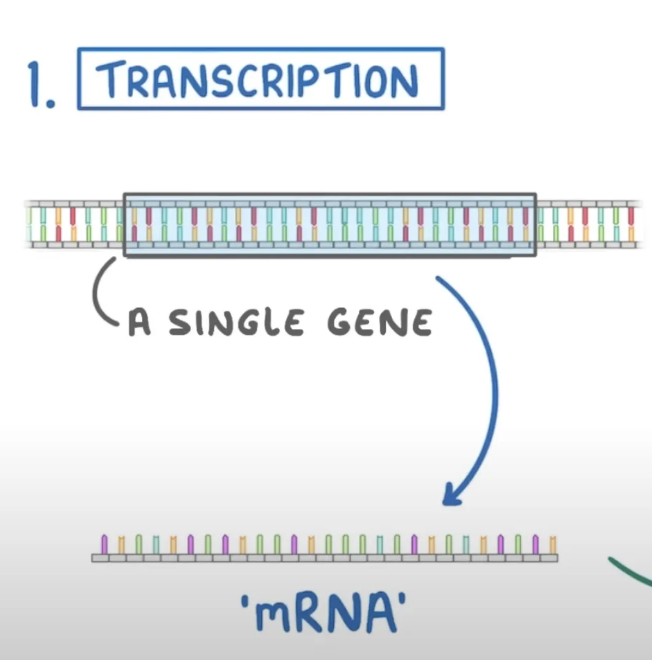
Formation of mRNA (stage 1 of transcription)
RNA polymerase binds to the DNA just before the gene starts.
the DNA around the gene unzips, separating the strands and exposing the bases.
one DNA strand acts as the template strand.
RNA polymerase lines up free RNA nucleotides complementary to the template strand to form the mRNA strand.
Stage 2 of transcription
once the mRNA strand is formed, it detaches itself from the DNA template and the DNA zips back up.
the mRNA strand exits the nucleus via a nuclear pore and travels to a ribosome for translation.
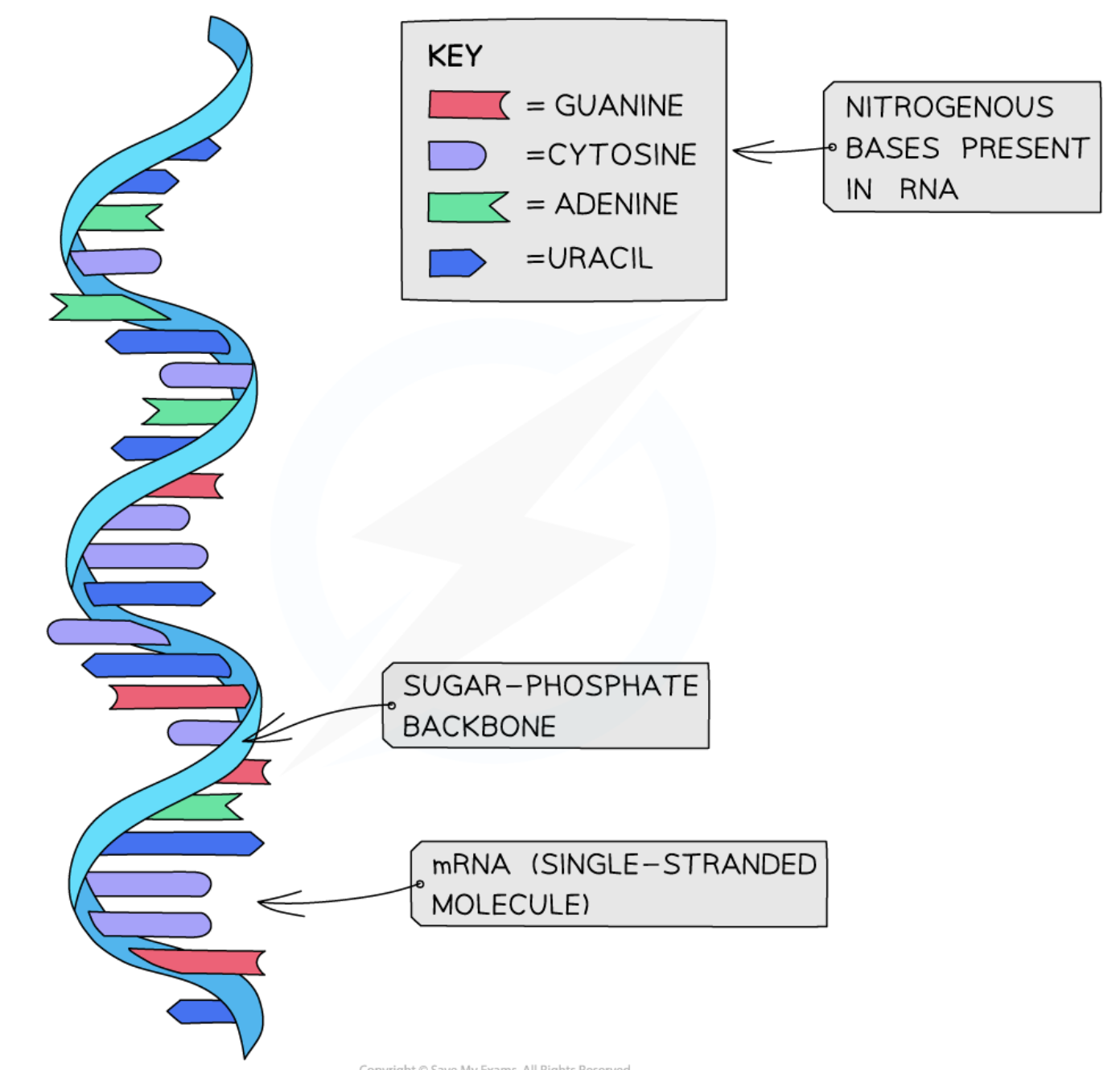
The base which replaces T in mRNA
Uracil
Why mRNA is important
DNA molecules are too large to leave the nucleus, which is why mRNA is required.
Translation
the process by which proteins are made at ribosomes.
Translation stage 1
mRNA attaches to a ribosome.
the ribosome ‘reads’ the nucleotides on the strand in groups of 3 (codon).
each codon codes for a specific amino acid.
the ribosome continues to read the codons, adding more and more amino acids to a chain.
this chain is a protein.
The importance of the sequence of amino acids in a protein
determines how the protein will fold; each type of protein has a specific shape which is important for protein function.
Transcription & translation
Enzymes
biological catalysts (speed up the rate of a reaction) that do not get used up in a reaction.
Two types of enzyme-controlled reactions
Anabolic - building larger molecules from smaller ones
Catabolic - breaks larger molecules into smaller ones
Do enzymes bind to many different types of substrate molecules?
nah - they’re highly specific, and only bind to one type of substrate molecule.
Lock and key hypothesis

Factors affecting enzymes
temperature
pH level
enzyme concentration
substrate concentration
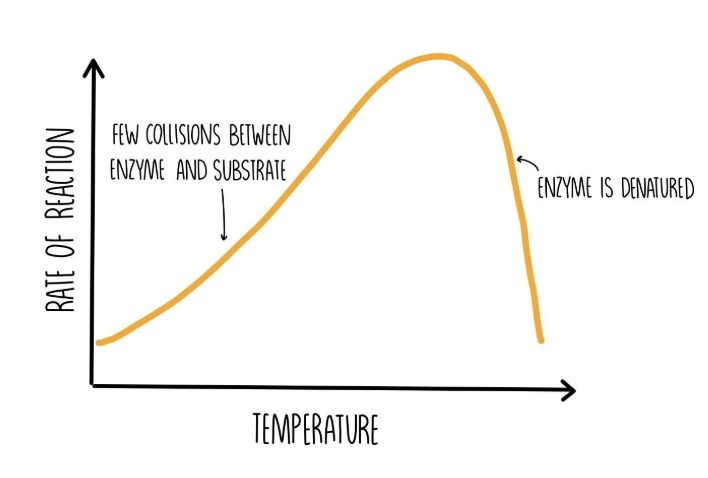
Effect of temperature on enzyme-controlled reactions
at higher temperatures the enzymes have more kinetic energy, increasing the frequency of successful collisions. the rate of reaction is faster.
at very high temperatures the enzymes’ active sites change shape, preventing them from binding to substrate molecules. this is denaturation.
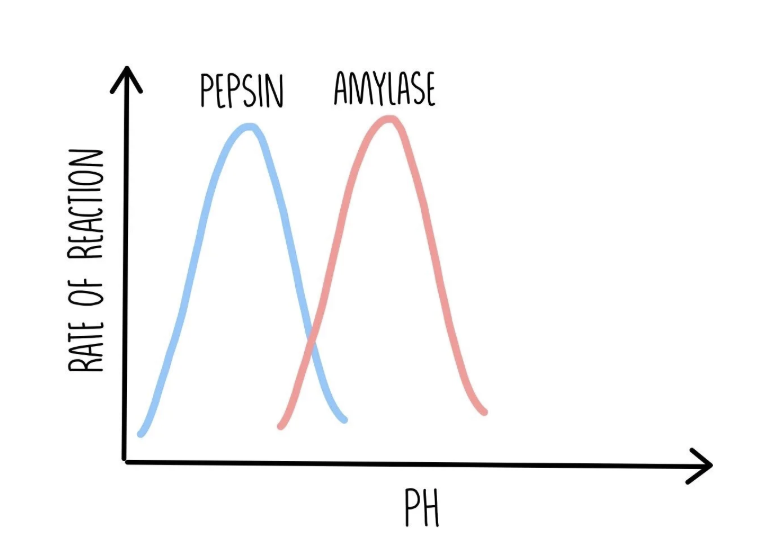
Effect of pH level on enzyme-controlled reactions
every enzyme works best at a certain pH level (optimum pH).
if the pH level varies too much, the bonds holding the amino acid chain together may be broken or disrupted, changing the active sites’ shapes, preventing them from binding to substrate molecules. this is denaturation.
Effect of enzyme concentration on enzyme-controlled reactions
an increase in enzyme conc. increases the rate of reaction, because more active sites are available to bind to substrate molecules (increased frequency of collisions).
after a certain point, the rate of reaction doesn’t increase, as the enzyme conc. is no longer a limiting factor.
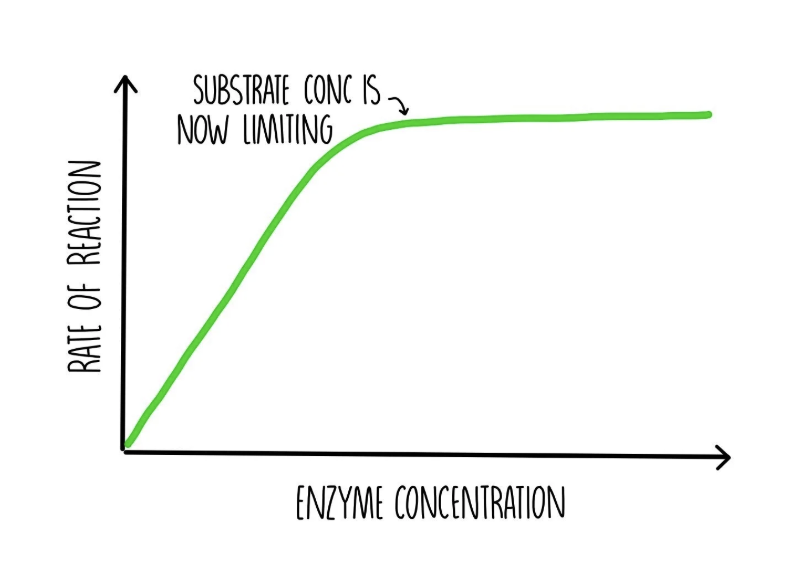
Effect of substrate concentration on enzyme-controlled reactions
an increase in substrate conc. increases the rate of reaction, because more substrate molecules are available to bind to active sites (increased frequency of collisions).
after a certain point, the rate of reaction doesn’t increase, as all the active sites are saturated. the only way to increase rate of reaction is to increase enzyme conc.

Cellular respiration
the process of transferring energy from the breakdown of glucose. it is a universal chemical process continually occurring in all living cells.
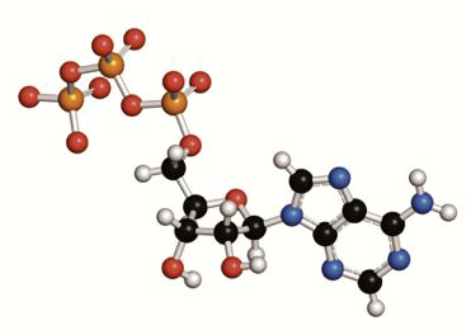
How is energy from cellular respiration stored?
in the form of ATP.
Why do organisms require energy?
chemical reactions (to synthesise larger molecules from smaller ones)
movement (ATP enables muscles to contract)
warmth (constant temp. for enzyme activity)
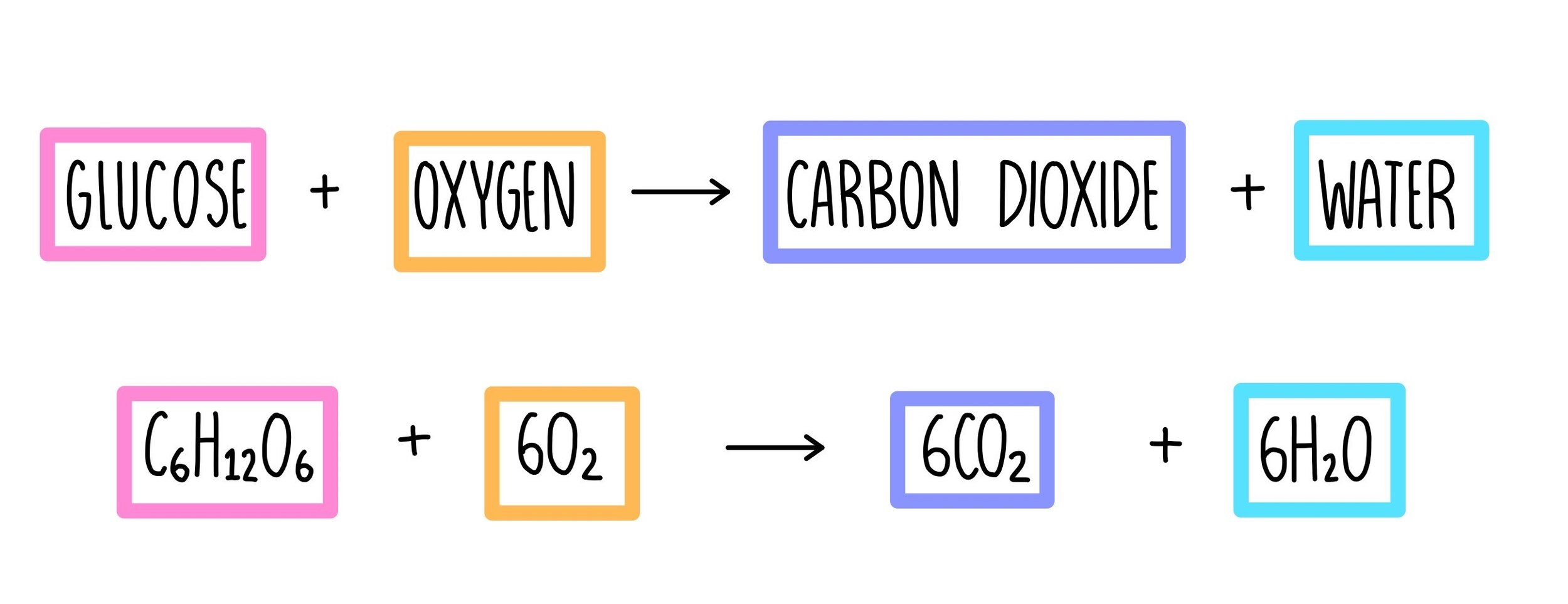
Aerobic respiration
requires oxygen
completely breaks down glucose molecules to release a relatively large quantity of energy.
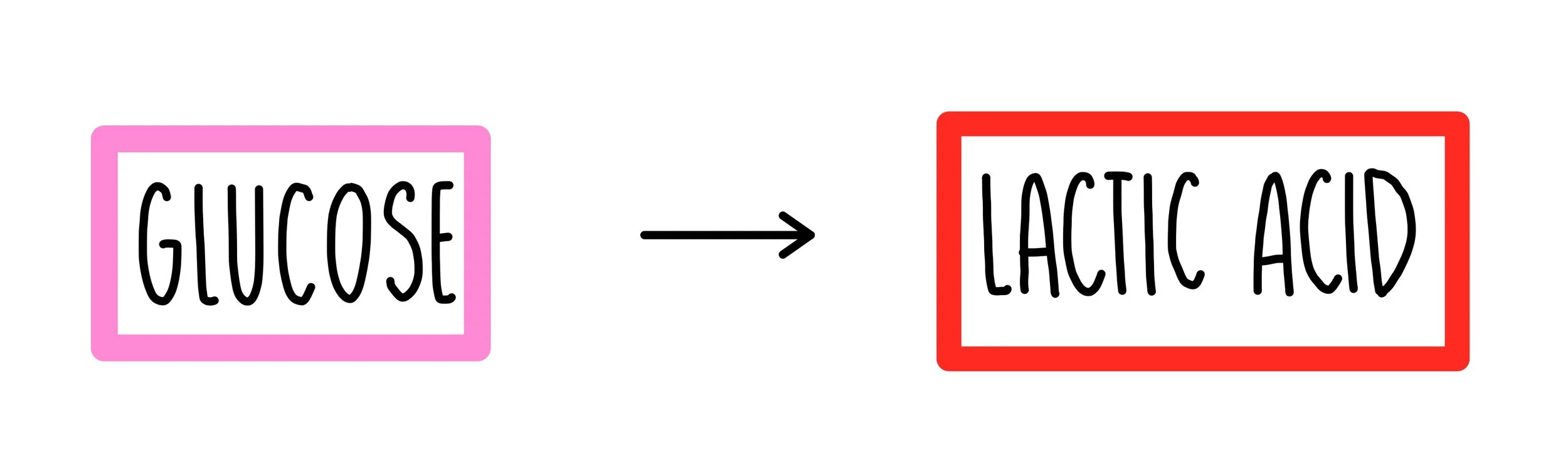
Anaerobic respiration
does NOT require oxygen
the incomplete break down of glucose molecules releases significantly less energy compared to aerobic respiration.
Why does anaerobic respiration occur?
during strenuous exercise, muscles need to release more energy than normal
our heart rate & breathing rate cannot increase fast enough to supply muscle cells with enough oxygen
glucose is not completely oxidised, releasing less energy quicker.
Why do we normally respire aerobically?
aerobic respiration produces more ATP molecules per glucose molecule than anaerobic respiration.
lactic acid from anaerobic respiration can cause cramps. the build up of lactic acid in muscle cells causes pain, and the muscles stop contracting - fatigue.
Oxygen debt
the quantity of extra oxygen required to break down the lactic acid which has built up.
Anaerobic respiration in microorganisms and plant cells
fermentation
occurs in anerobic conditions ✌😅 (e.g. in the roots of plants in waterlogged soils)

Metabolic rate
the rate at which all chemical reactions in the body occur.
Different groups of carbohydrates
monosaccharides - simple sugars consisting of a single monomer unit (e.g. fructose, glucose)
disaccharides - consist of two monosaccharides joined together (e.g. maltose is formed from two glucose molecules)
polysaccharides - lots of monomer units join together in a long chain to form a polymer (e.g. starch is formed when lots of glucose molecules join together).
Enzyme digesting carbohydrates
carbohydrase; however, amylase is used to break down starch
Proteins
synthesised from different sequences of amino acids.
there’s 20 different types of amino acids
Enzyme digesting proteins
protease
Lipids
synthesised from 3 fatty acid molecules & 1 glycerol molecule
Enzyme digesting lipids
lipase
Test for sugars
Benedict’s test
positive test = dark red/orange
negative test = remains blue
Test for starch
Iodine test
positive test = blue-black
negative test = orange-brown
Test for protein
Biuret test
positive test = purple / violet
negative test = blue
Test for lipids
Emulsion test
postive test = cloudy emulsion ☁
negative test = no change (colourless)
Photosynthesis
the process by which autotrophs use light energy, CO2 & H2O to produce glucose.
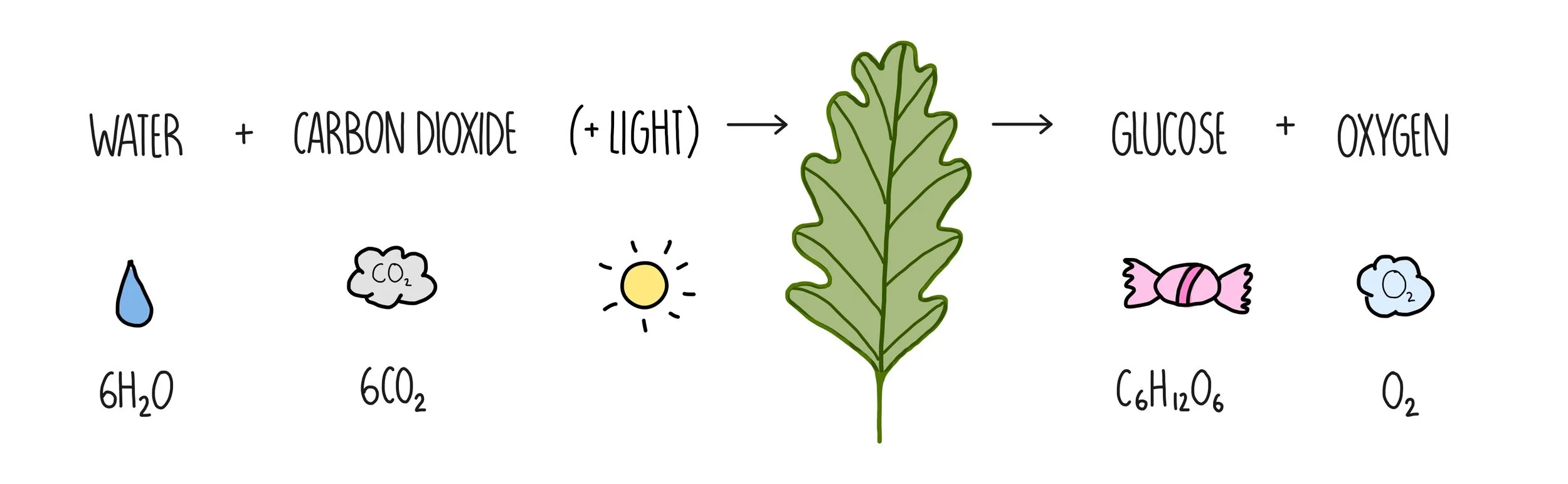
Photosynthesis equation
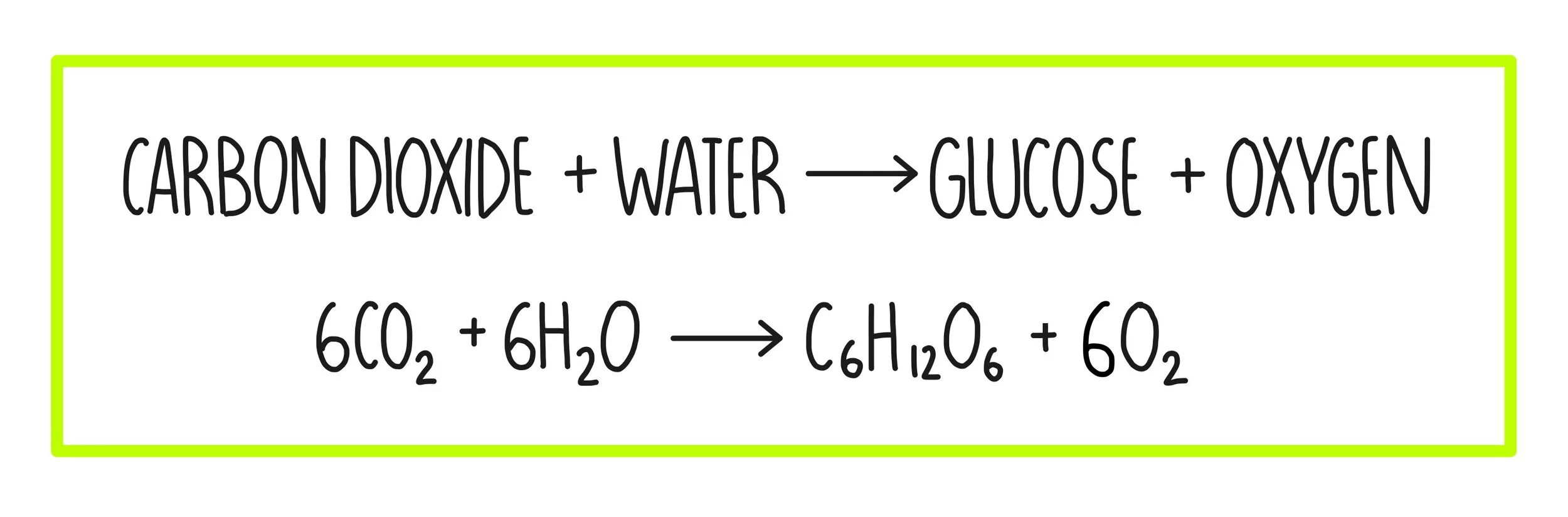
Site of photosynthesis
chloroplasts in leaves (which contain chlorophyll). the pigment absorbs sunlight, where the energy is used to react CO2 & H2O to produce C6H12O6.
Stage 1 of photosynthesis
energy transferred from light splits water molecules into oxygen gas & hydrogen ions.
Stage 2 of photosynthesis
carbon dioxide combines with the hydrogen ions to make glucose.
Uses of glucose produced in photosynthesis
converted to starch to provide an energy store for respiration at night
converted into cellulose to form cell walls
Limiting factors of photosynthesis
carbon dioxide
temperature
light intensity
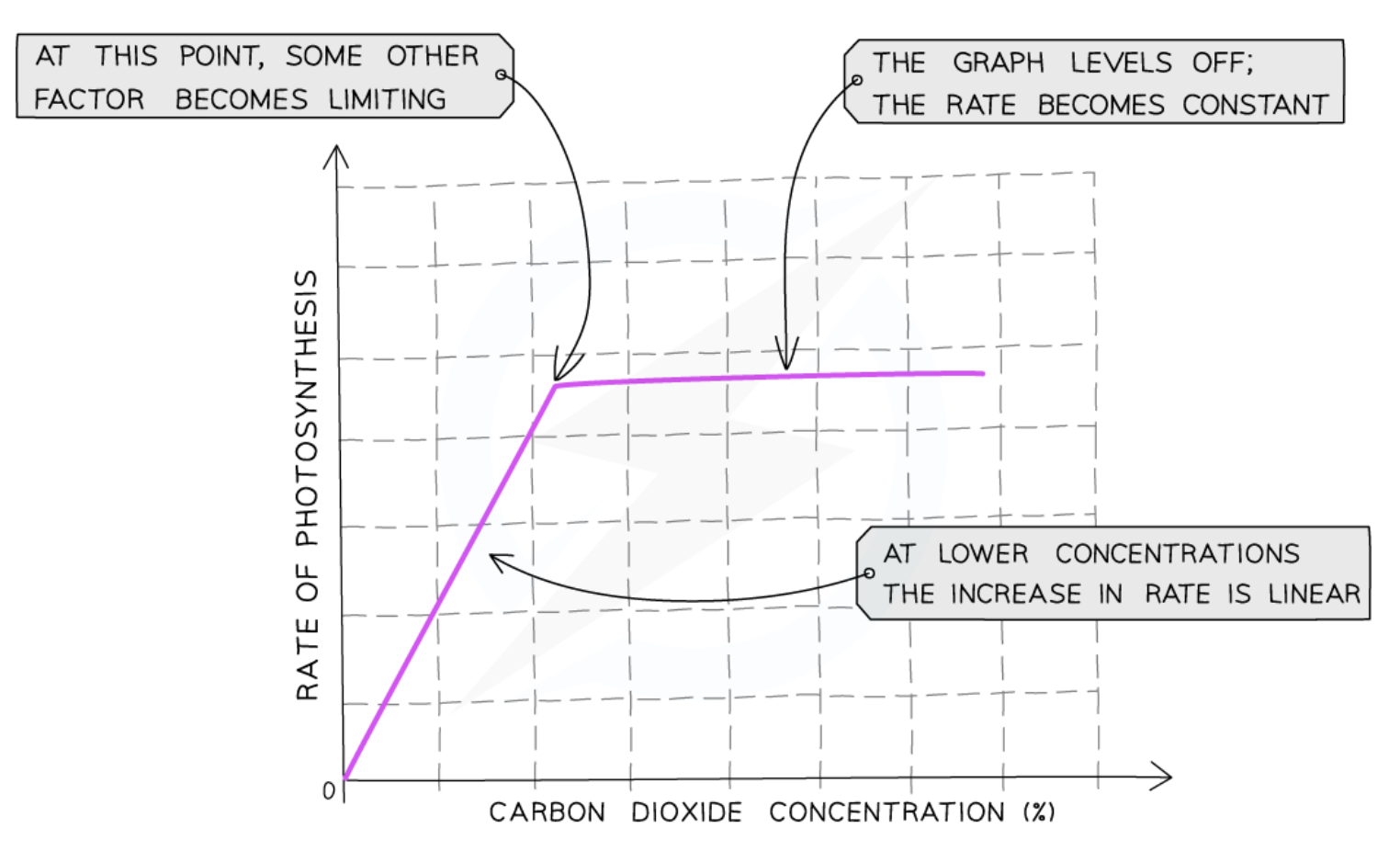
Effect of carbon dioxide concentration on rate of photosynthesis
the more CO2, the faster the rate of photosynthesis until a certain point
once that point is reached, some other factor prevents the rate of photosynthesis increasing further
CO2 is no longer the limiting factor
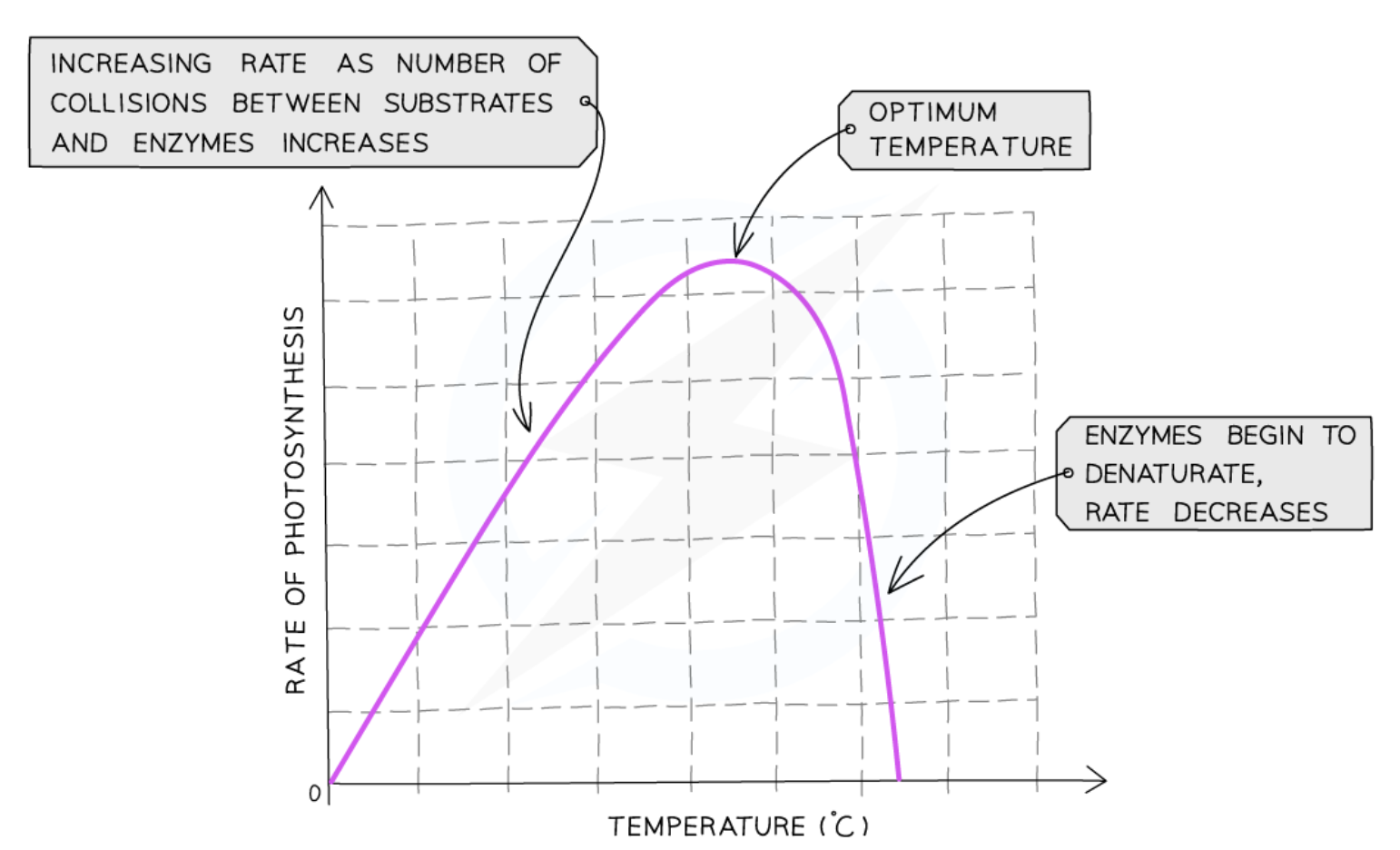
Effect of temperature on rate of photosynthesis
increasing temperature = increasing kinetic energy of reactant particles
frequency of successful collisions increases
at very high temperatures, enzymes controlling photosynthesis denature
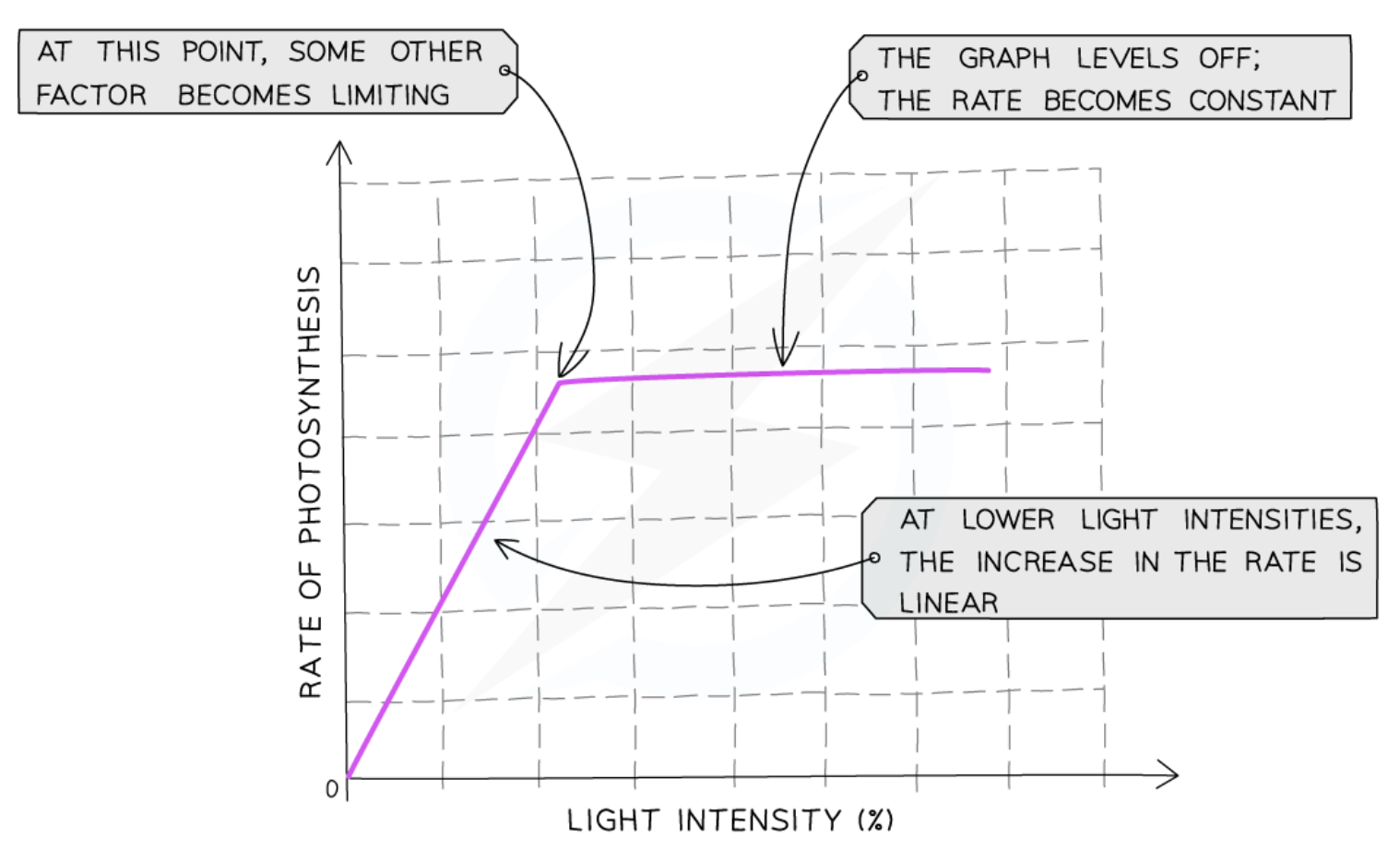
Effect of light intensity on rate of photosynthesis
the more light a plant receives, the faster the rate of photosynthesis until a certain point
once that point is reached, some other factor prevents the rate of photosynthesis from increasing further
light intensity is no longer a limiting factor
The inverse square law
