Biology VCE unit 3 and 4
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Biomolecules
Molecules which are produced by living organisms and are involved in the biological functions of cells.
There are 4 main classes;
-Proteins
-Carbohydrates
-Nucleic Acids
-Lipids
Monomers and Polymers
Nearly all biomolecules consists of building blocks known as _____ which form ______.
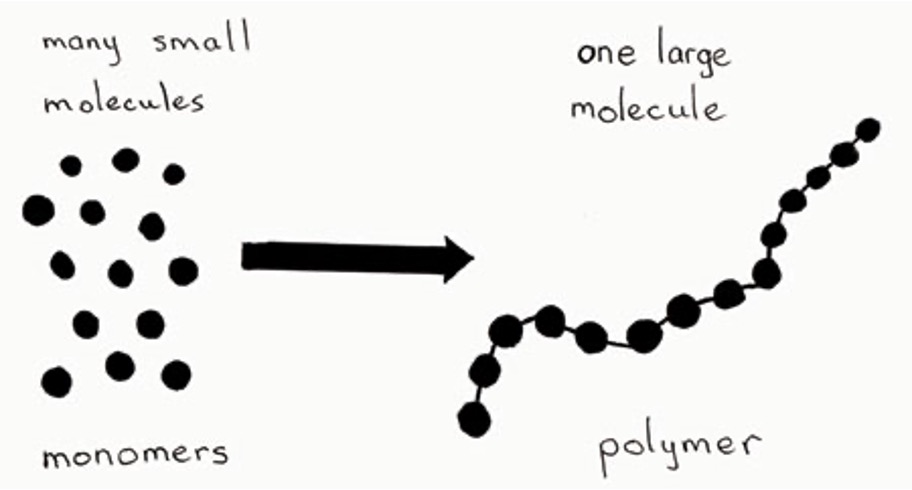
What are they made of?
Each biomolecule is based off of a carbon chain, however each biomolecule has unique elements and characteristics.
Carbohydrate: CHO = carbon, hydrogen, and oxygen.
Lipid: CHO = carbon, hydrogen, and oxygen.
Protein: CHON = carbon, hydrogen, oxygen, and nitrogen.
Nucleic acid: CHONP = carbon, hydrogen, oxygen, nitrogen, and phosphorus.
Carbohydrate
Monomer = simple sugars/ monosaccharides
Monosaccharide is one unit, Disaccharide is two units, and Polysaccharide is multiple units.
Supply energy for cells and form structures.
example of this are glucose (simple sugar for energy), starch (energy storage in plants), glycogen (energy storage in animals), and cellulose (structural component of plant cell walls).
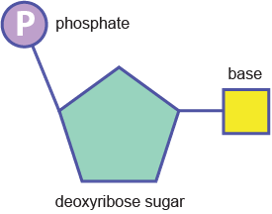
Nucleic acid
Monomer = Nucleotide
Encode the genetic information for cells to create proteins. Present in the forms of DNA & RNA.
examples of this are DNA and RNA.
The picture show the circle as the phosphate, the square as the base and the other shape as the sugar. the picture is showing a deoxyribose sugar phosphate. You know if it is deoxyribose or ribose by looking at the base, if there is two then it is ribose, if there is one the it is deoxyribose.

DNA
double-stranded, deoxyribose as the sugar, bases used are Thymine (T), Cytosine (C), Adenine (A), and Guanine (G). G = C and A = T.
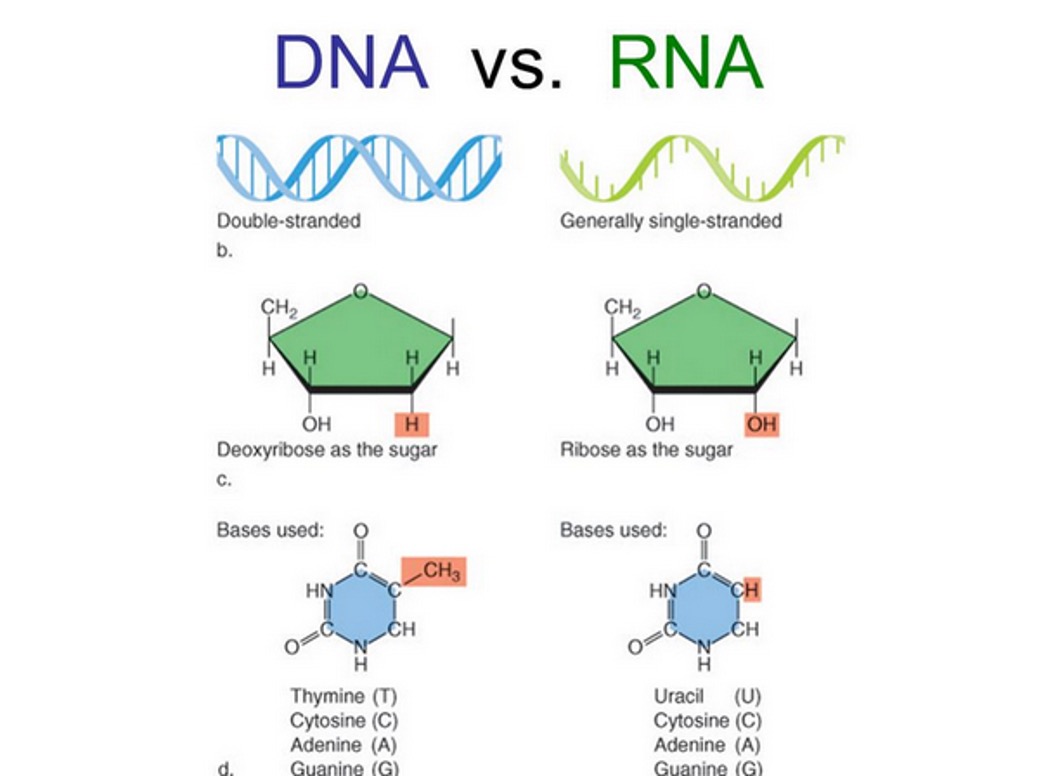
RNA
Generally single-stranded, ribose as the sugar, bases used are Uracil (U), Cytosine (C), Adenine (A), and Guanine (G). Uracil replaces. Thymine. G = C and A = U.
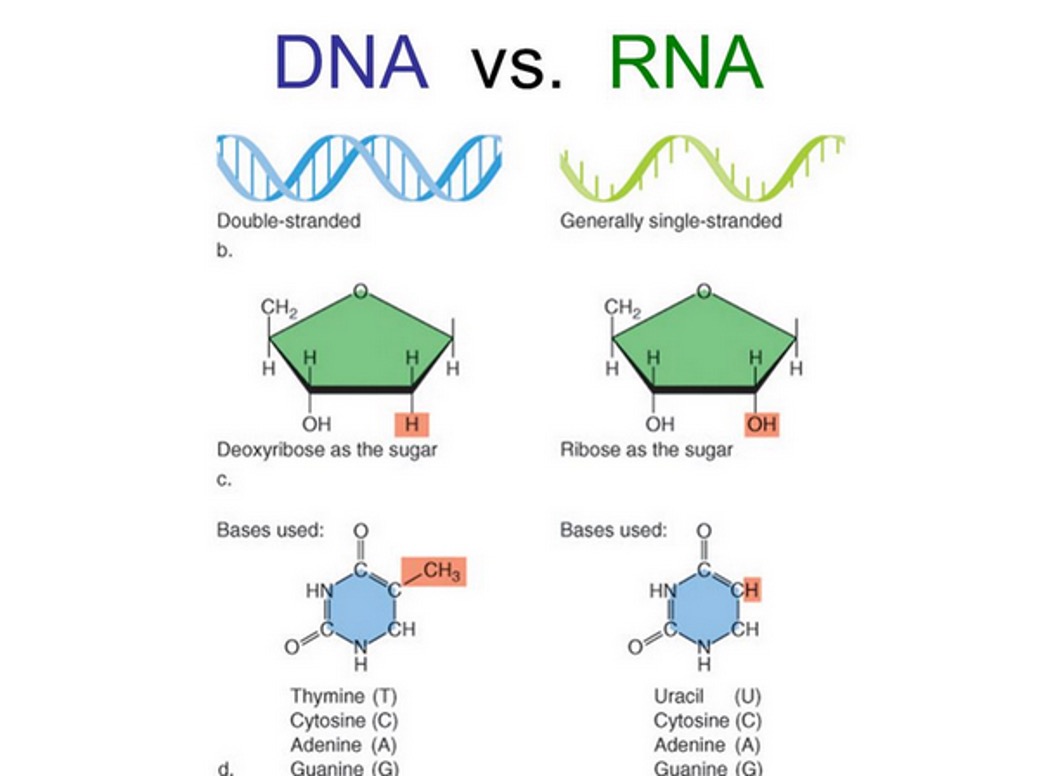
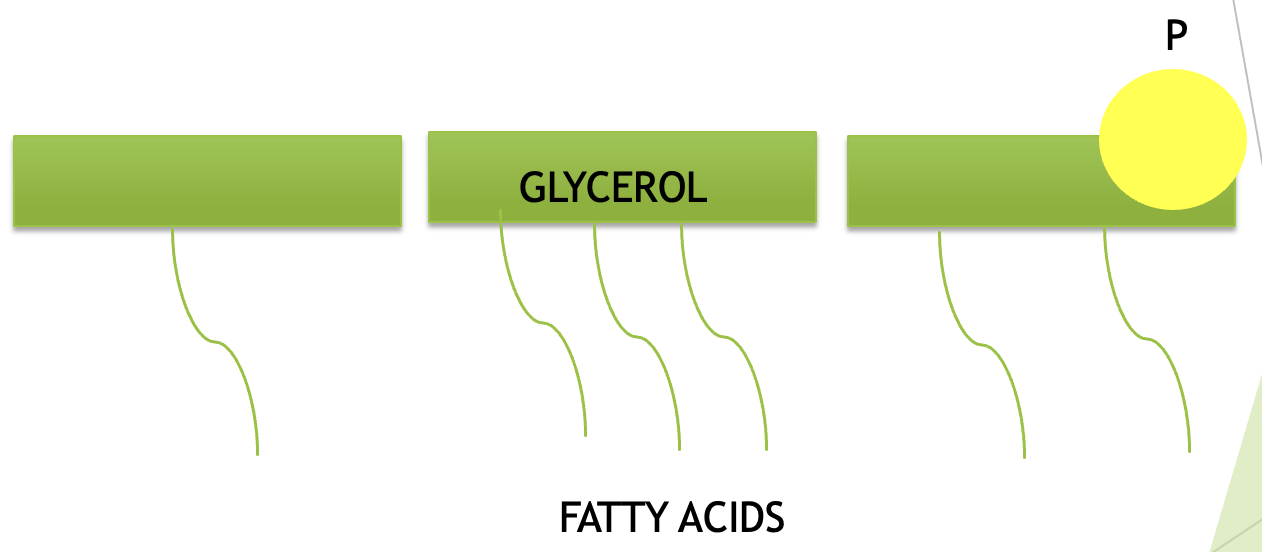
Lipid
Monomer = Nearly all lipids contain a glycerol and fatty acids.
Involved in forming membranes, storing energy and signalling.
examples of this are fats, oils, waxes, phospholipids, and steroids.
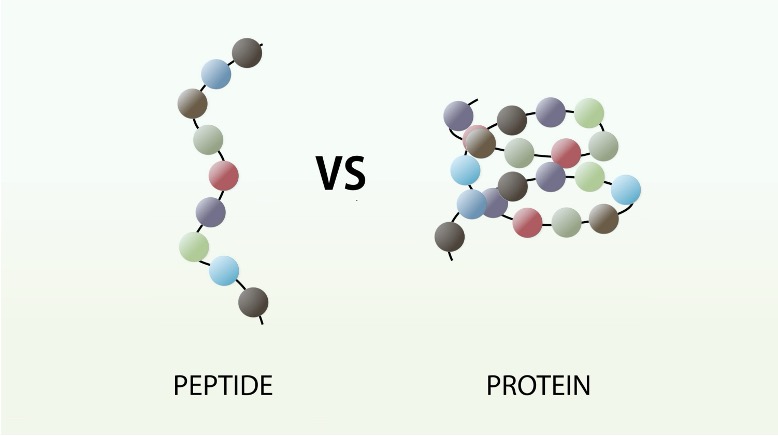
Proteins
Polypeptide = chain of amino acids
Monomer = a amino acid
Form enzymes, receptors, membrane components, hormones, structural supports, antibodies.
a biomolecule which are formed from long chains of amino acids known as polypeptides. The bonds formed between amino acids are known as 'peptide' bonds, hence the name 'Poly' - 'Peptide'. They are extremely important in the structure and function of all cells.
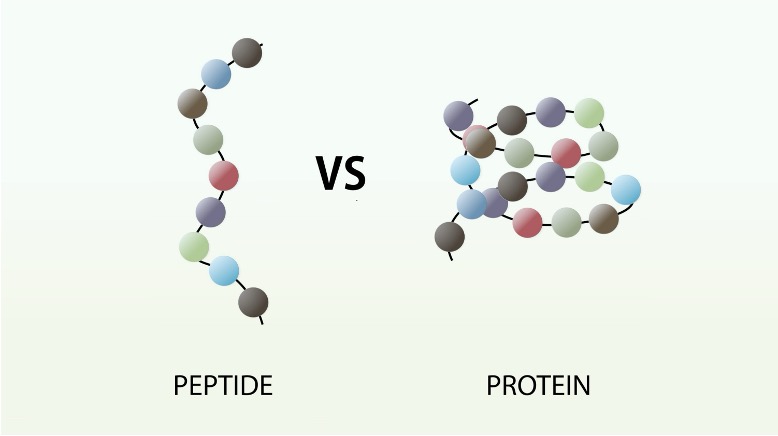
examples of proteins
enzymes (like Rubisco and catalase), structural proteins (like collagen, keratin, and actin), and transport proteins (like hemoglobin).
Polypeptide
single chain of amino acids joined by peptide bonds.
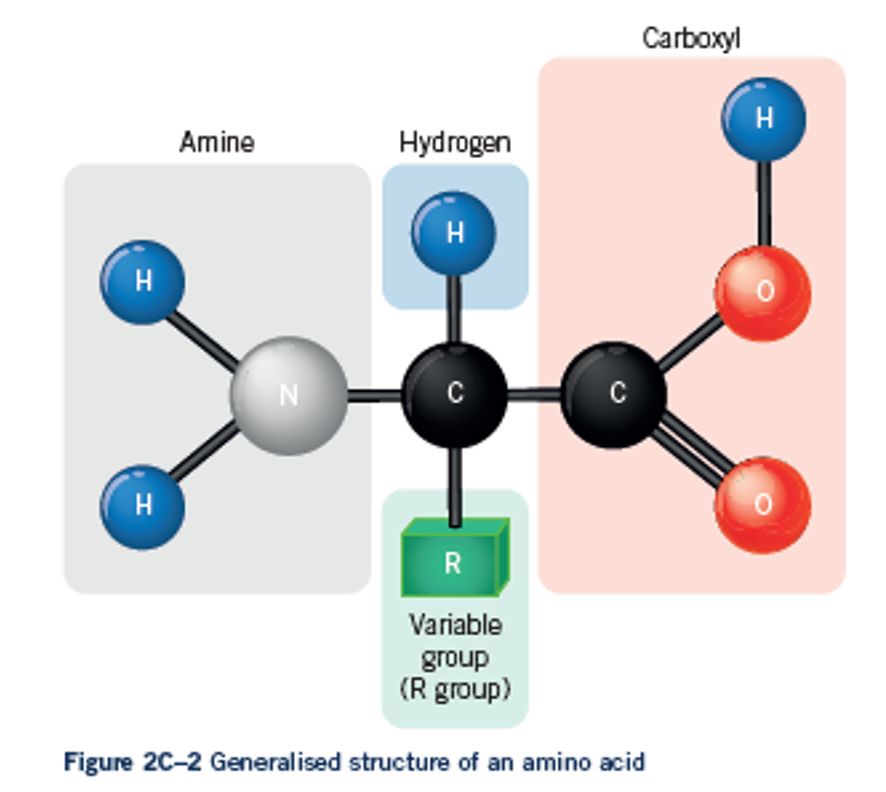
Amino Acids
are the building blocks of proteins. They are made of an amine group, a carboxyl group and an R group. The R group changes between each amino acid and this is what makes them unique.
You don’t need to know how to draw them, but you should know they contain these groups and Nitrogen (N).
Proteome
It is the complete set of proteins expressed by an organism. It is far larger than the genome (the total amount genes an organism can express). Each individual cell expresses the proteins they need. E.g. Heart cells express different proteins to a skin cell. This allows them to save energy and become specialised.

Types of Proteins
Proteins can be categorised as fibrous or globular.
Fibrous
used only as structural components.
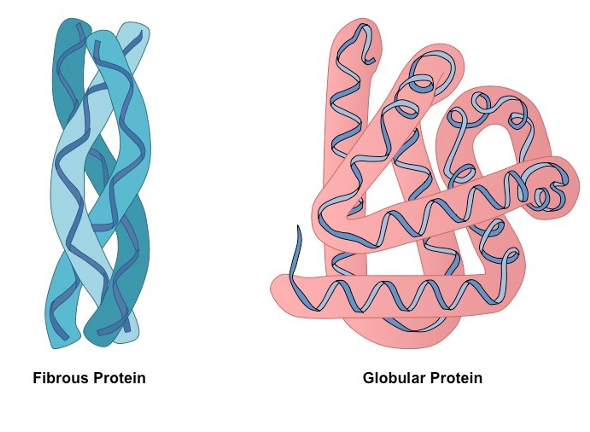
Globular
used in the function of the cell (e.g. enzymes and hormones).

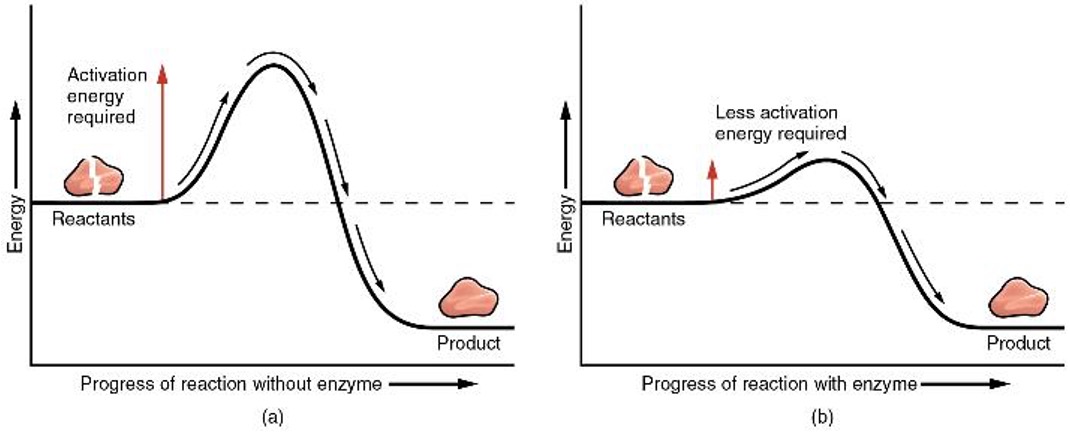
Enzymes
proteins which catalyze (speed up) reactions by lowering the activation energy of the reaction. They are highly specific and their shape matters. If a word ends in ase it is likely to be an enzyme like amylase (digests starch), lipase (digests fats), protease (digests proteins), and lactase (digests lactose in milk).
How do they work?
binding to reactants in such a way that the bond breaking or building process happens more readily. This lowers the activation energy required for reactions to take place.
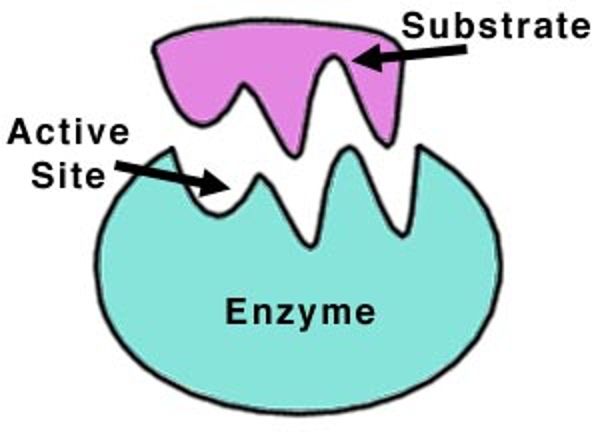
Active site
Region of an enzyme where the reaction occurs. This is usually complimentary in shape to the substrate.
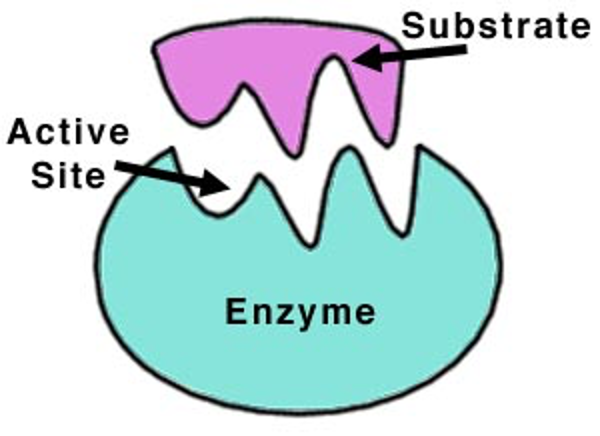
Substrate
a substance on which an enzyme acts.
The induced fit model
Enzymes bind to the substrate via this. This means the active site slightly changes to bind to the substrate.
Anabolic Reactions (Endergonic)
Enzymes can combine substrates into a single product. They need energy for this.
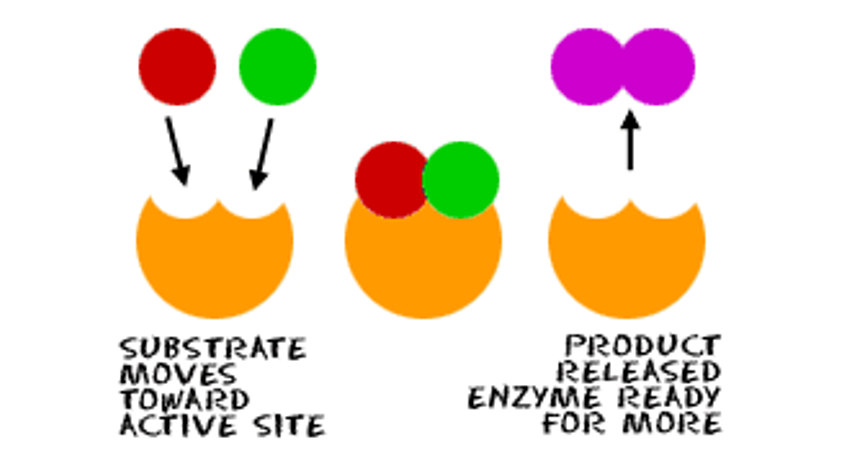
Catabolic Reactions (Exergonic)
Enzymes can also break a single substrate into multiple products. Energy is released.
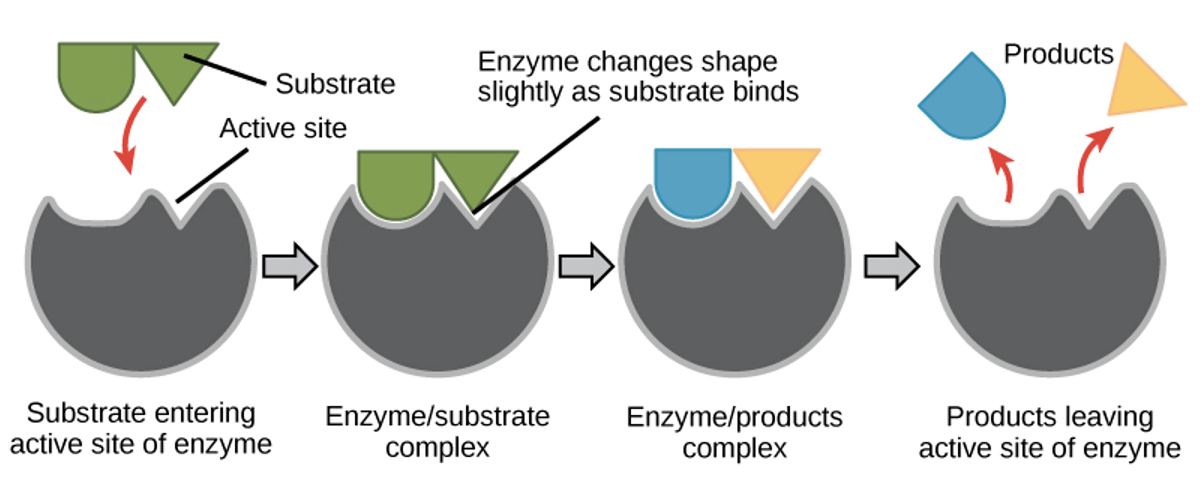
How enzymes work
Enzymes lower the activation energy of a reaction and make it easier for reactants to combine.The rate at which they work can be affected by a range of factors including temperature, pH, enzyme concentration, and substrate concentration.
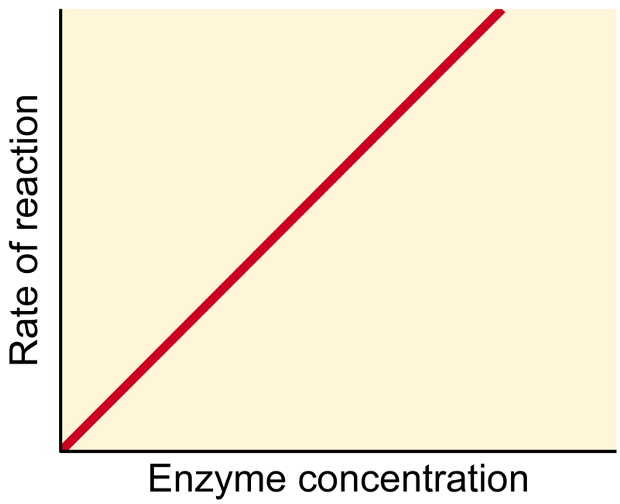
Enzyme Concentration
Increasing in it will continually increase the rate of reaction as long as there is unlimited substrate.
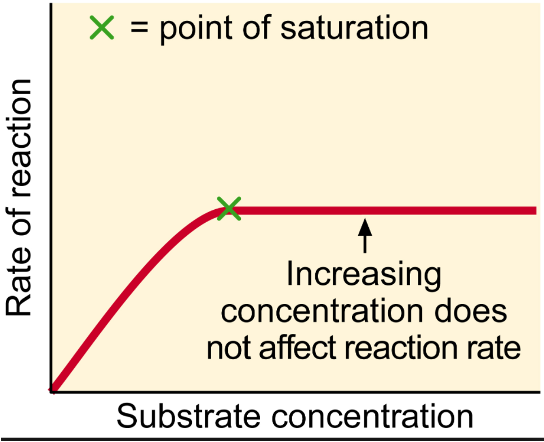
Substrate Concentration
If no additional enzymes are added, increasing in it will increase the rate of reaction until all the enzymes are occupied by a substrate. This is called the ‘point of saturation’.
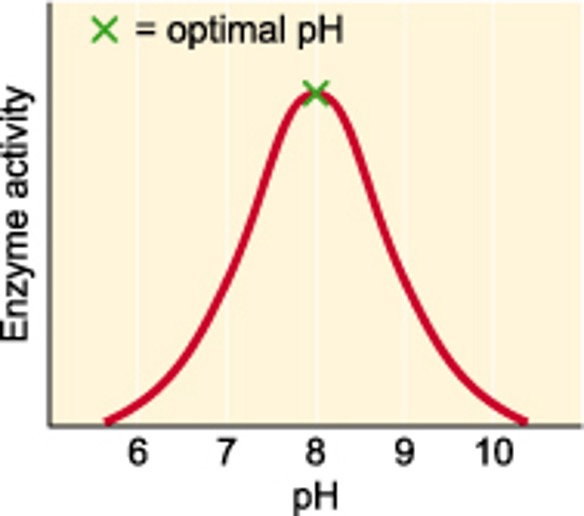
pH
Enzymes work at an optimal of this. This optimal changes depending on the enzyme. Either side of this the enzyme will not function as effectively.
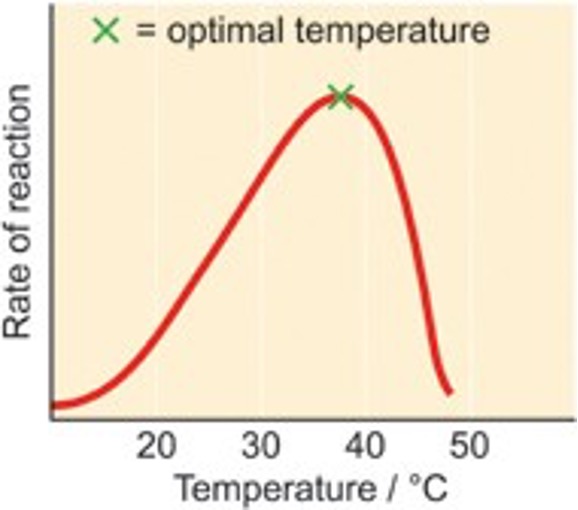
Temperature
Enzymes also work at an optimal of this. The optimal changes depending on the enzyme. An increase in this means the enzyme and substrate has a higher kinetic energy and collides more often.
Impact of Factors
If an enzyme is not at an optimal pH or too high of a temperature it may begin to denature and lose its shape.
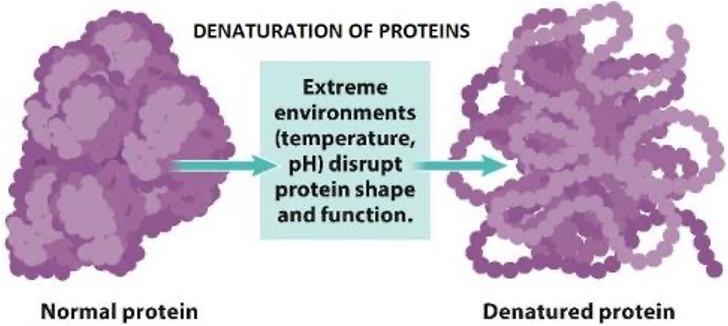
Denaturation
Destruction of characteristic properties of a molecule by heat or pH.
Enzyme Inhibition
Enzymes can be slowed by molecules known as inhibitors. Depending on how strong the inhibitor binds, they can be reversible or irreversible.
Covalent bonds = irreversible inhibition.
Hydrogen bonds = reversible inhibition.
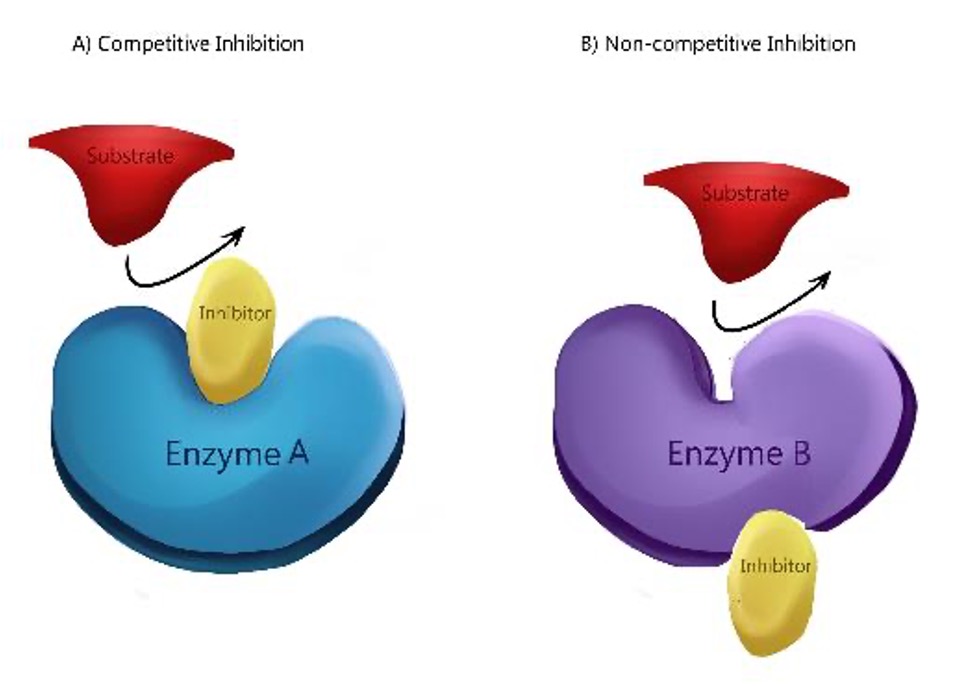
Competitive Inhibition
Block the active site of an enzyme and prevent the active site and substrate from binding.
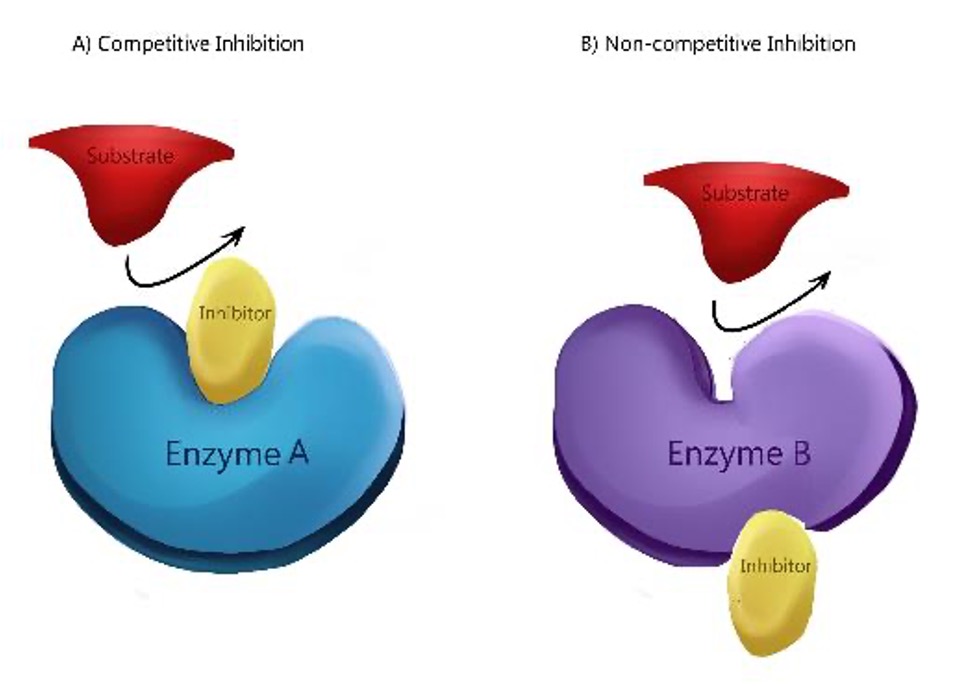
Non-Competitive Inhibition (sometimes called allosteric inhibition)
The inhibitor binds at another site and causes the active site to change shape. The alternative site is called the allosteric site.