3.10.1 bonding
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
aromatic compound
a cyclic, planar organic molecule with a continuous ring of delocalised pi electrons
this delocalisation gives the molecule extra stability
benzene
an aromatic cyclic hydrocarbon consisting of a ring of 6 carbon atoms with 6 hydrogen atoms, and a central ring of delocalised pi electrons
C6H6
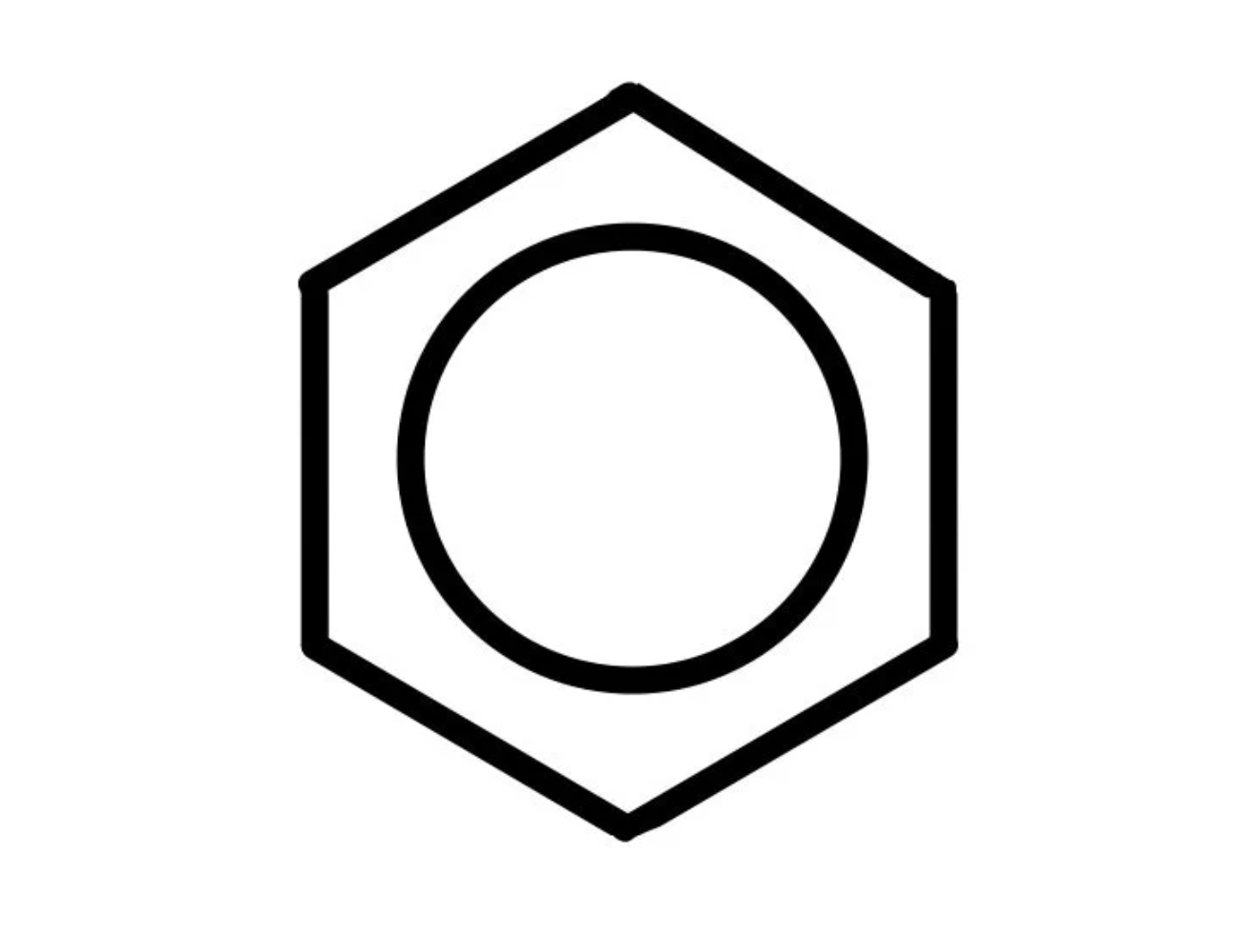
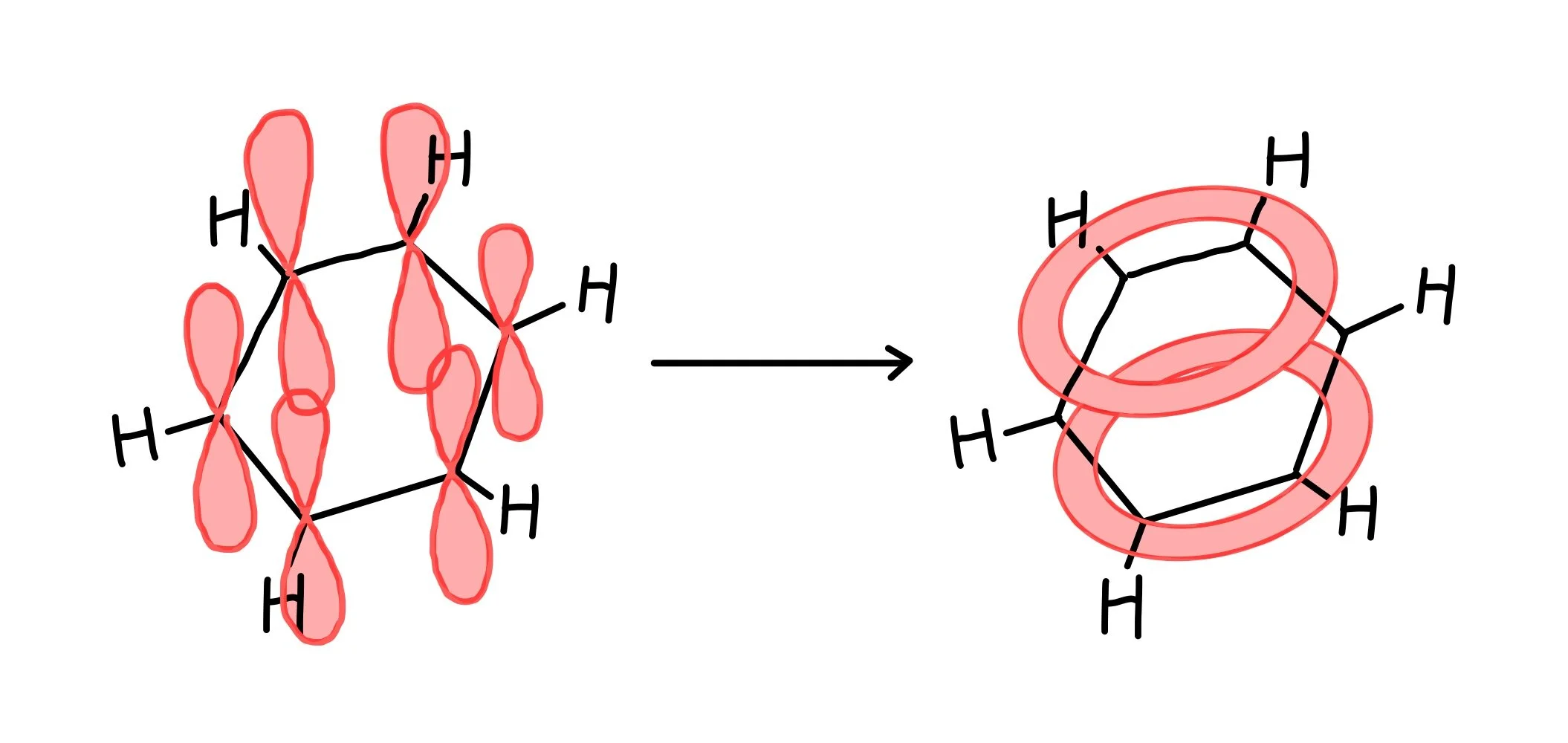
describe the nature of bonding in a benzene ring
planar structure, all bond angles are 120°
each bond in the ring is the same length and has an intermediate length between a single and a double C to C bond
the outer electron from the p-orbital of each C atom is delocalised. these p-orbitals overlap sideways to form a continous delocalised ring above and below the ring of C atoms.
the delocalised electrons form pi bonds spread around the ring of C atoms
delocalisation of p electrons makes benzene more stable
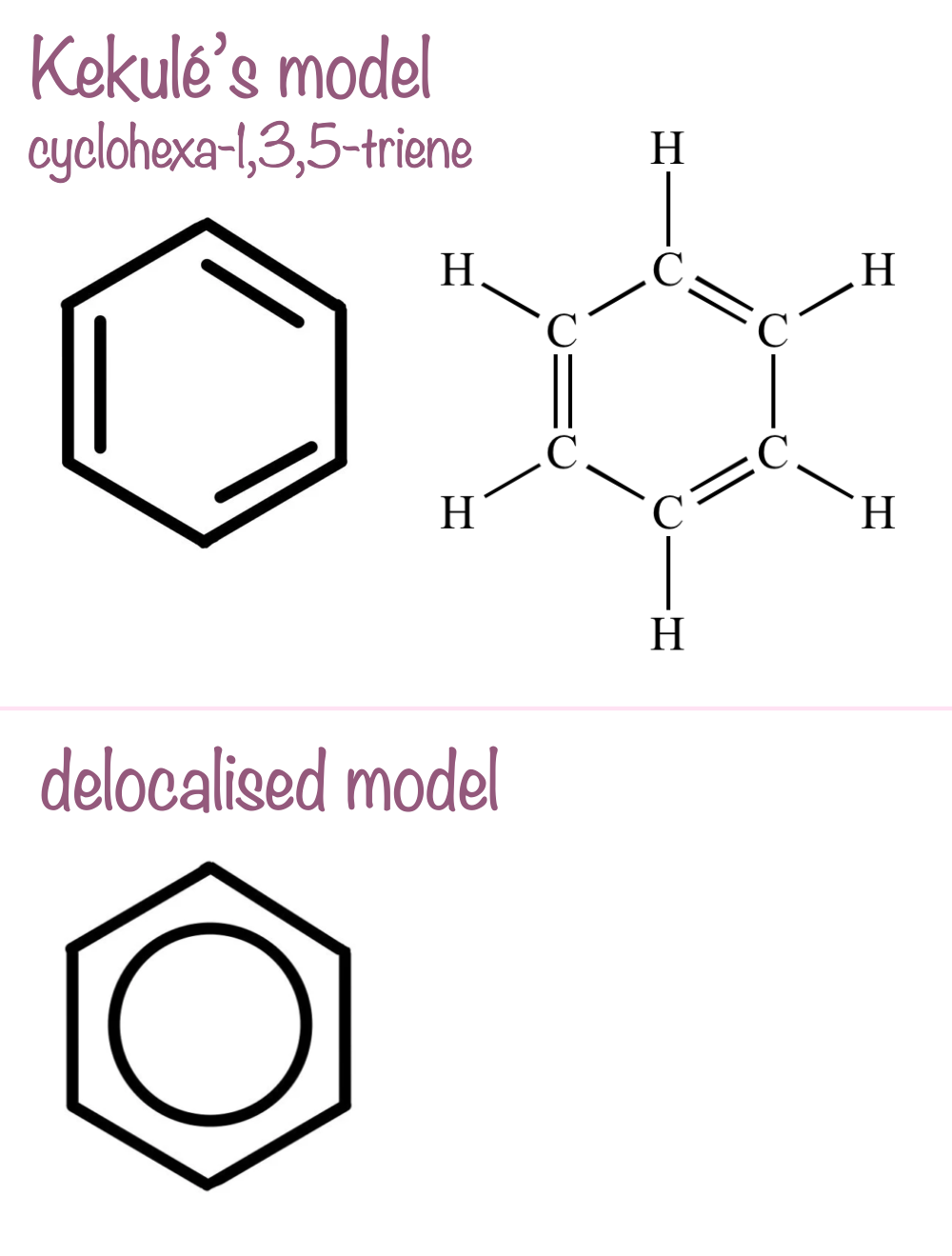
what was Kekulé’s proposed benzene structure?
he suggested cyclohexa-1,3,5-triene, which has alternating single and double bonds in its ring (3 of each bond type)
this structure was predicted from empirical measurements
how does the reactivity of benzene disprove Kekulé’s proposed benzene structure?
if benzene contained double bonds, it would react like an alkene
benzene does not carry out electrophilic addition and does not decolourise bromine water
so, benzene does not contain double bonds
how do the bond lengths in benzene disprove Kekulé’s proposed benzene structure?
C-C bonds are longer than C=C bonds. if benzene contained both single and double C to C bonds, it would have bond of different lengths in its ring.
benzene is perfect regular hexagon. it has equal C to C bond lengths and this bond length is an intermediate between single and double.
so, benzene does not contain a mix of C-C bonds and C=C bonds.
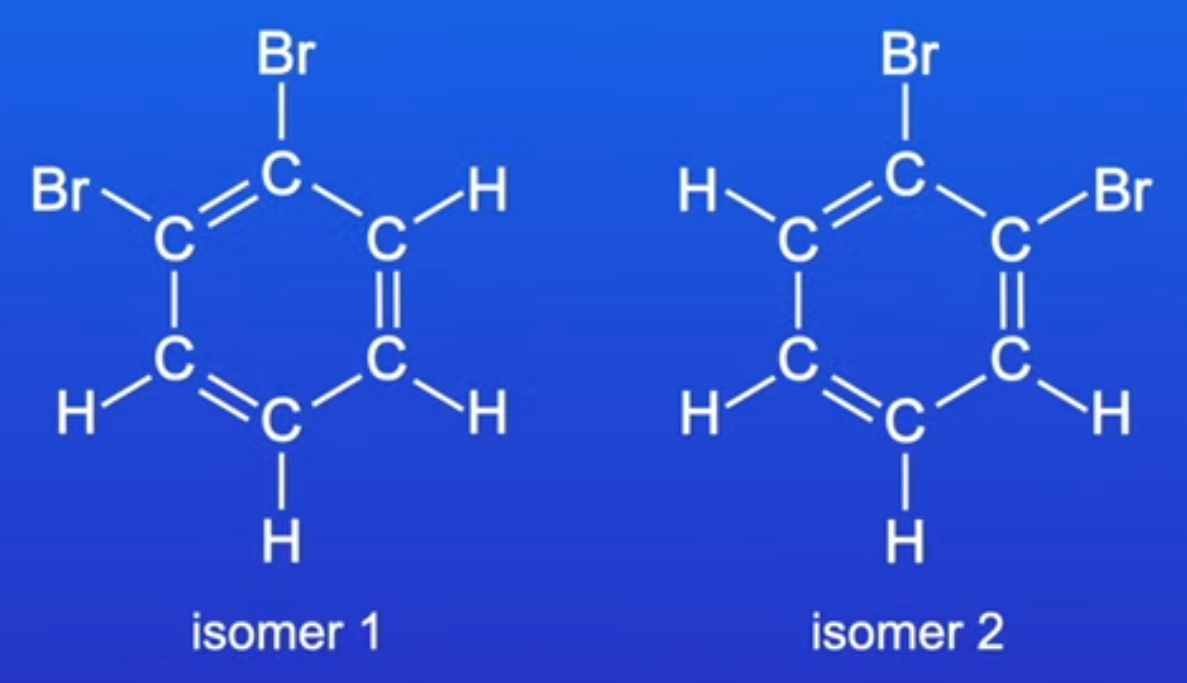
how does isomerism disprove Kekulé’s proposed benzene structure?
if benzene had the cyclohexa-1,3,5-triene structure, it would have 2 isomers of 1,2-dibromobenzene. isomer 1 would have a Br atom on either side of the C=C bond, and isomer 2 would have a Br atom on either side of the C-C bond.
these isomers cannot be made.
so, Kekulé’s proposed structure is incorrect.
use thermochemical evidence from enthalpies of hydrogenation to:
disprove Kekulé’s proposed benzene structure, and
compare the stability of benzene and the theoretical molecule cyclohexa-1,3,5-triene
cyclohexene evolves 120 kJ mol-1, so expect cyclohexa-1,3,5-triene to evolve 3 x 120 = 360 kJ mol-1
360 - 208 = 152 kJ mol-1, the enthalpy of hydrogenation of benzene is 152 kJ mol-1 less exothermic
due to delocalisation of p electrons in benzene
so benzene is more stable than cyclohexa-1,3,5-triene
what was correct about Kekulé’s proposed benzene structure?
both models are planar/hexaganol
arenes
aromatic organic compounds that contain a benzene ring as part of their structure
benzene can become a side group: the phenyl group C6H5
the prefix ‘phenyl’ can be used when naming the compound
properties of arenes
high melting points due to high stability of the delocalised ring
low boiling points as they are non-polar - van der Waals forces between molecules are easily overcome
often insoluble in water
why doesn’t benzene react with bromine in the same way as alkenes?
in benzene, pi electrons are delocalised above and below the whole ring of C atoms
so, the electron density between any two adjacent C atoms is less than in the C=C bond in alkenes
so, benzene cannot polarise Br2 and induce a dipole
so, electrophilic addition does not occur
for benzene and bromine to react, we need halogen carier catalyst
why can benzene be attacked by electrophiles?
how does benzene react with electrophiles?
the delocalised ring is an area of high electron density which attracts electrophiles
benzene cannot induce an electrophile, so a catalyst may be used to generate an electrophile with a + charge
benzene undergoes electrophilic substitution
delocalisation energy
the energy required to break the pi bonding system of delocalised electrons in benzene
explain why electrophilic substitution reactions occur in preference to electrophilic addition reactions
to maintain the stable delocalised ring in the product
delocalisation energy is too high
benzene cannot induce an electrophile