Periodic Trends--MY
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Alkali Metals (group #)
group 1 on the periodic table.
Transition Metals (group #)
groups 3-12.
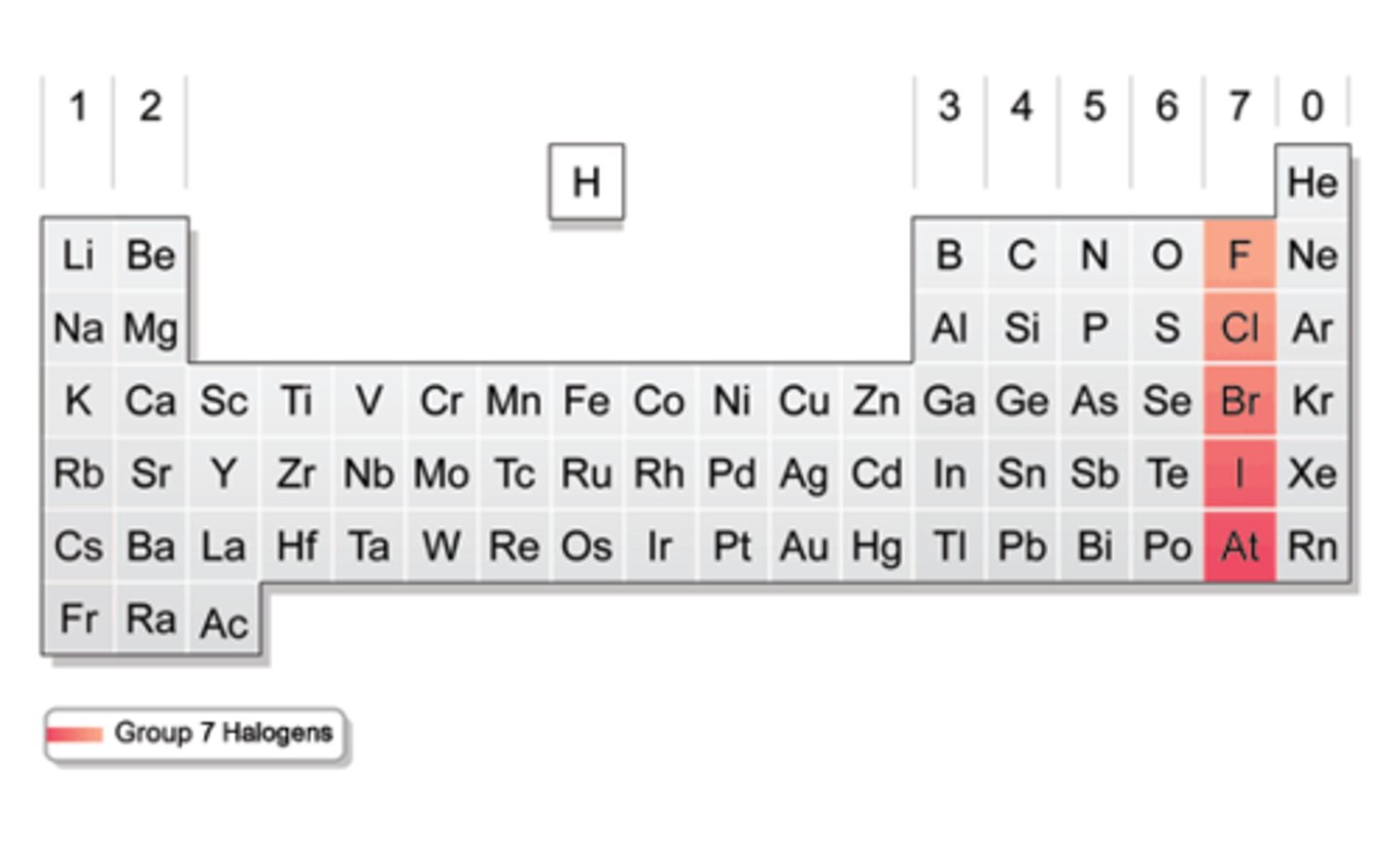
Halogens (group #)
group 17.
valence electrons (location in atom)
are found in the outermost energy level.
Noble Gases (group #)
group 18.
Electronegativity (definition)
tendency of an atom to attract/gain electrons
Atomic Radius (definition)
The size of an atom's electron cloud.
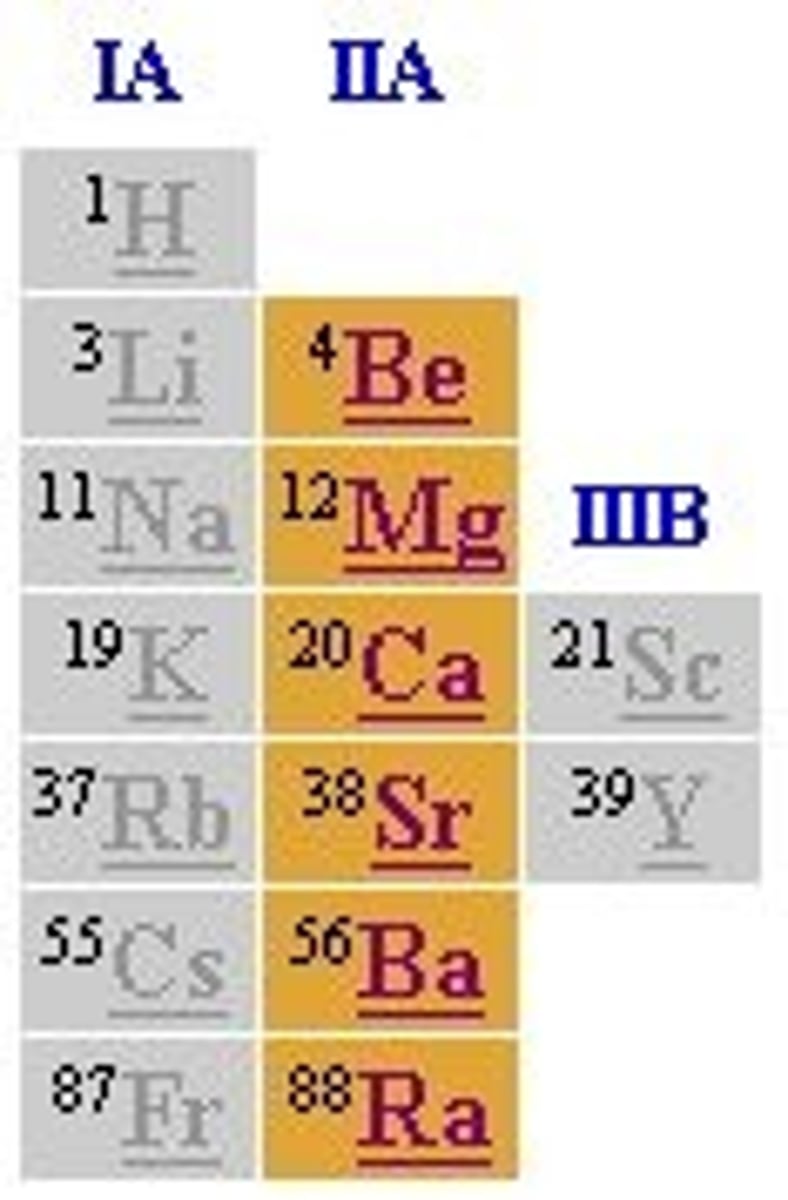
Alkaline Earth Metals (group #)
group 2.
2 valence electrons.
Metals (definition)
left of stairstep; tend to lose electrons/form cations.
Non Metals (definition)
tend to gain electrons/form anions.
upper right corner of periodic table.
Halogens (picture)
Alkaline Earth Metals (picture)
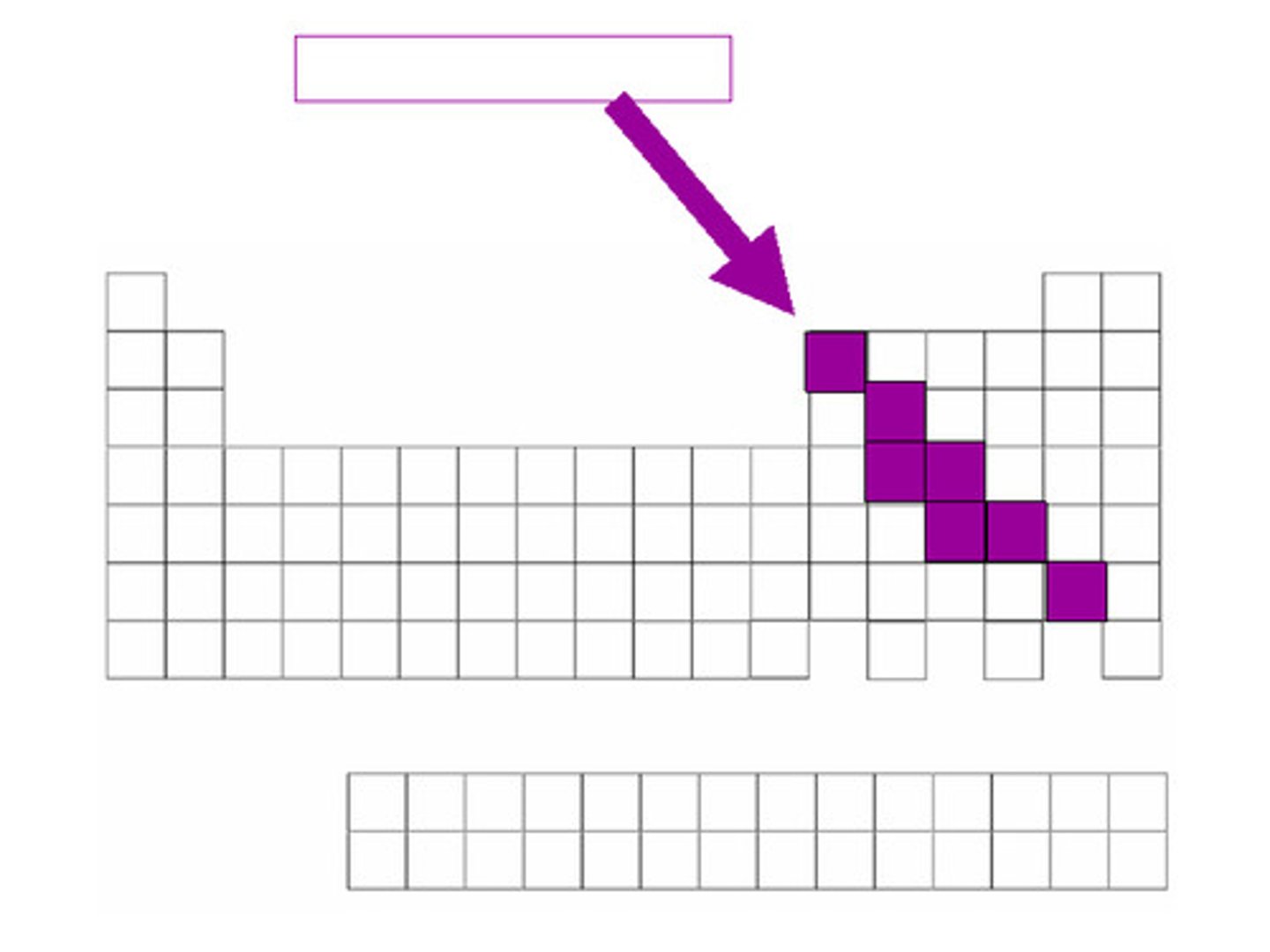
Metalloids (picture)
Non Metals (picture)
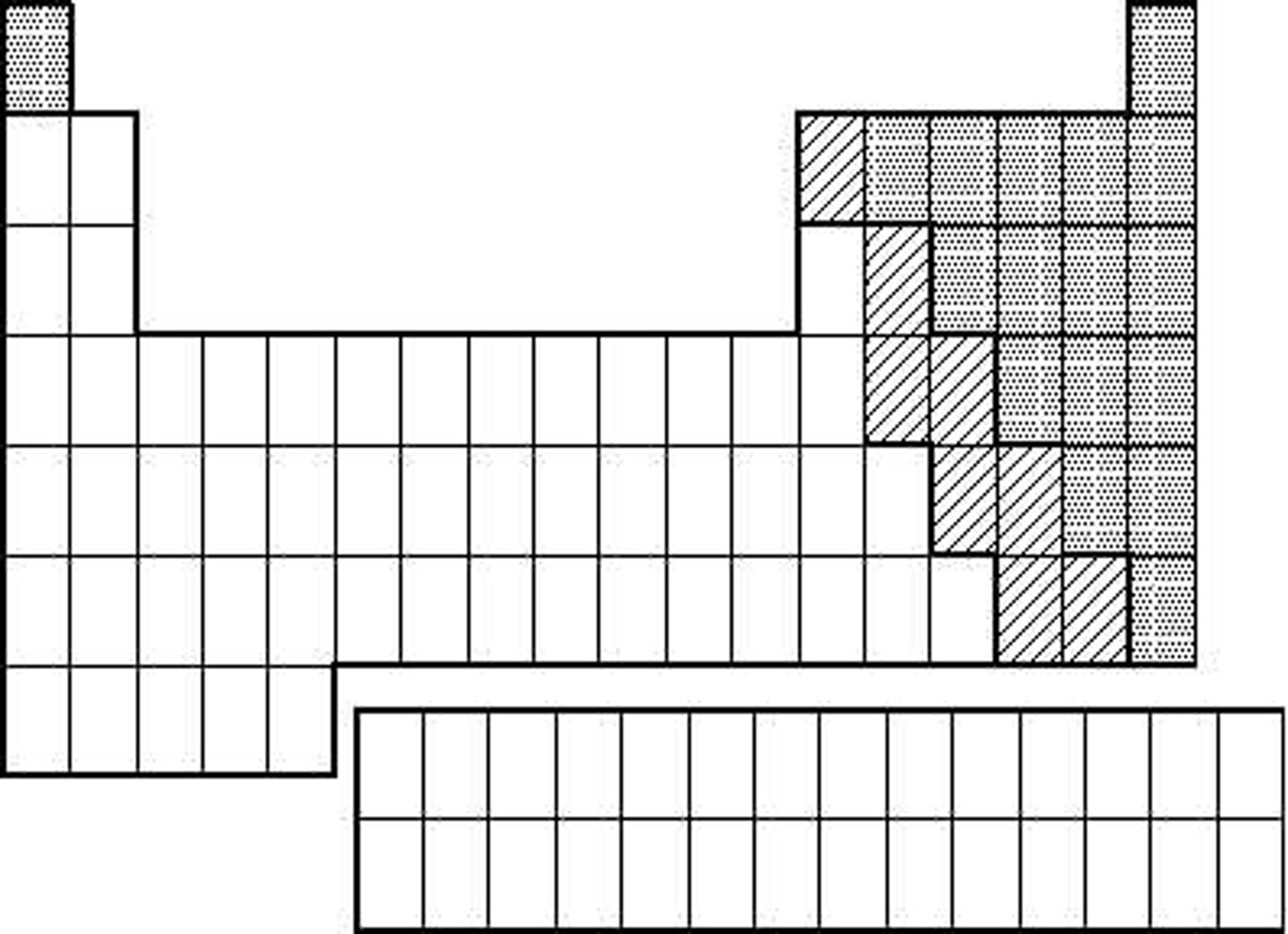
Transition Metals (picture)
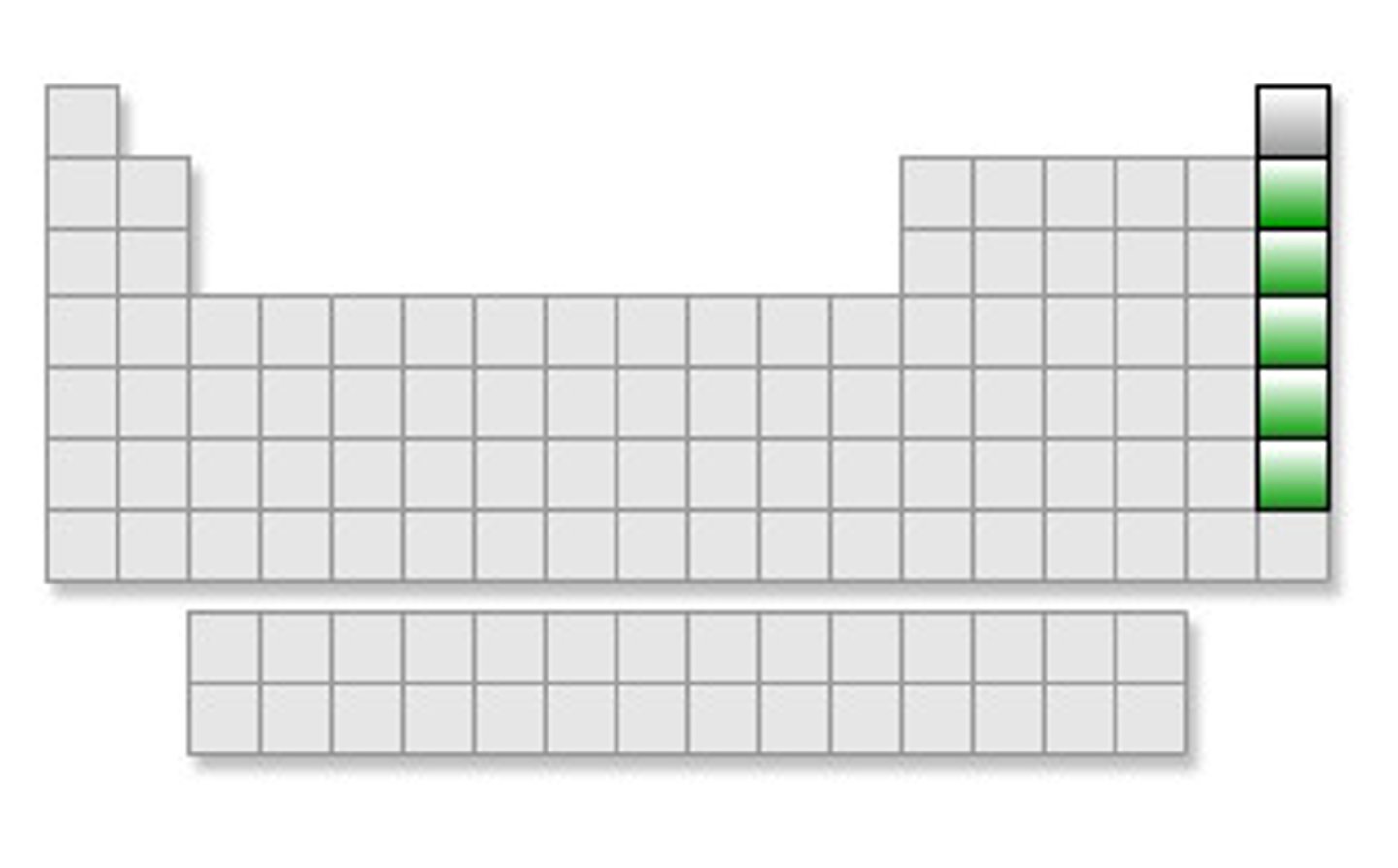
Noble Gases (Picture)
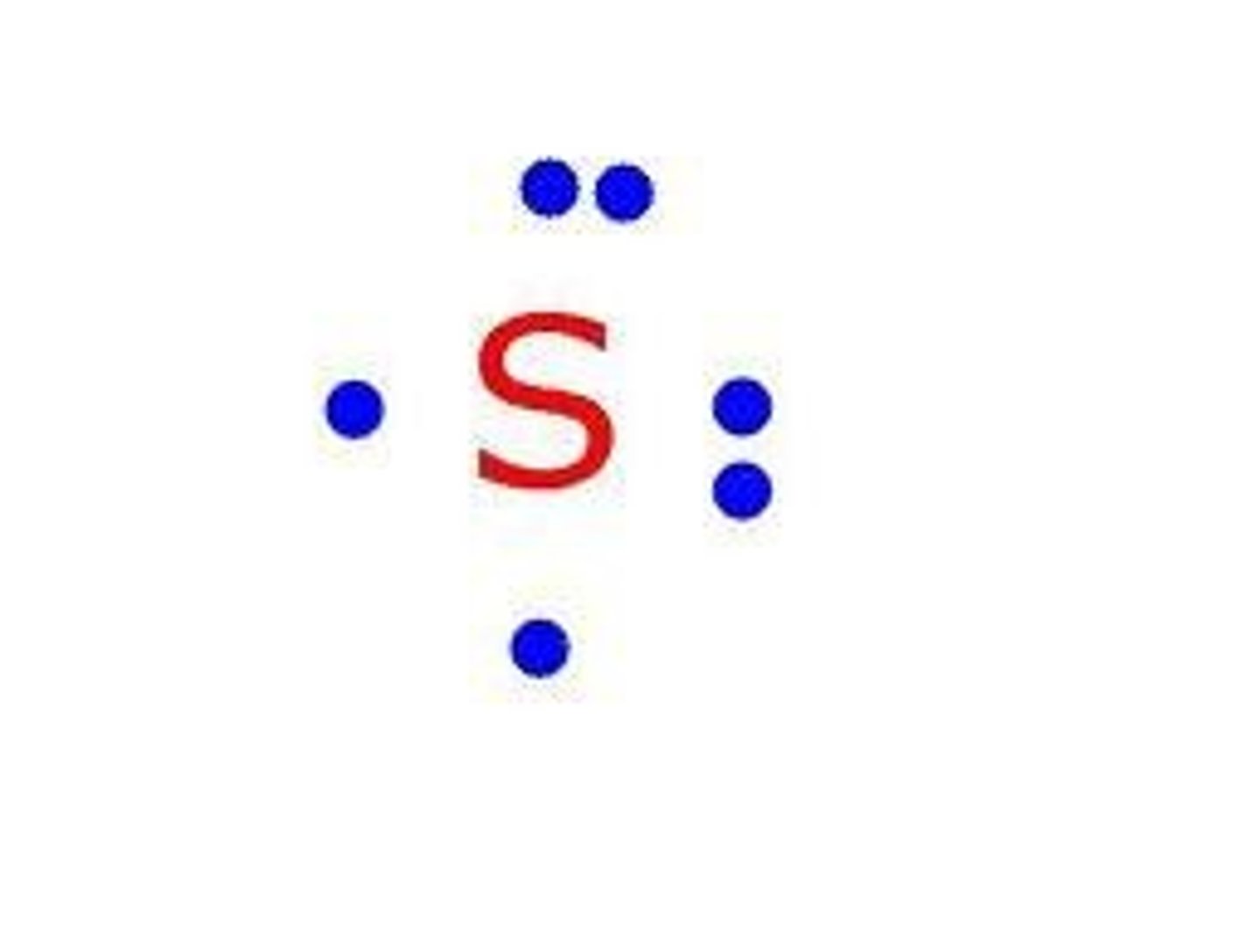
Electron (Lewis) Dot Diagram.
Shows valence electrons.
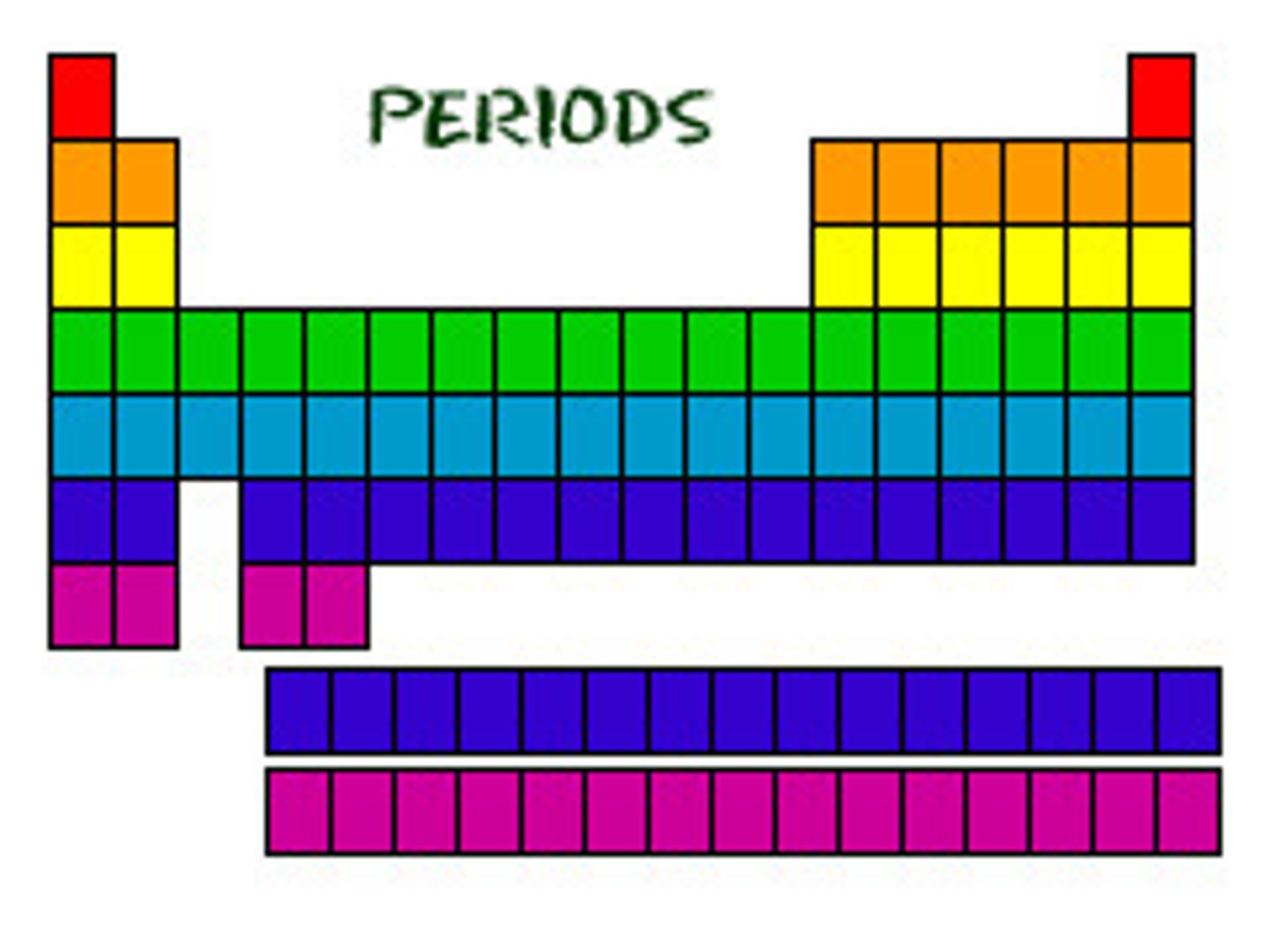
Periods/Rows. (definition)
From left to right across the periodic table.
Alkaline Earth Metals (valence electrons)
Family with two valence electrons.
Halogens (valence electrons)
Family with seven valence electrons.
Noble Gases (valence electrons)
Family with 8 valence electrons
Noble Gases (description)
Most stable and unreactive Family of elements.
Which of the following has the largest atomic radius (all in period 2): Li, B, N, F, Ne
Li
Which of the following has the highest ELECTRONEGATIVITY (all in period 2): Li, B, N, F, Ne
F
Which of the following is the LEAST REACTIVE element (all in period 2): Li, B, N, F, Ne
Ne
Which of the following elements wants to GAIN 3 electrons (all in period 2): Li, B, N, F, Ne
N
An increase in distance between a proton and electron (increases/ decreases) the force.
decreases (force of attraction)
The attractive force (increases/ decreases) as number of protons in the nucleus increases.
Increases (force of attraction)
Charge of the elements with 6 valence electrons.
Negative 2
Charge of the elements in family 1.
Positive 1.
What charge would an element be if it loses 2 electrons?
Positive 2
This is the charge an element with 7 valence electrons would form as an Ion.
Negative 1