ME 240 - Exam 1 Review - Timothy Becker NAU
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
BCC Crystals have detectable diffraction:
when the planes h+k+l = even
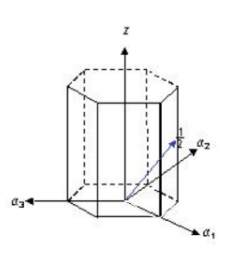
What is the correct miller indicies notation for the vector in the following hexagonal cell?
[2 2 4̅ 3]
The atomic weight of an atom is:
Number of protons and neutrons
Ionic bonds undergo:
electron transfer from one atom to another
Substitutional solid solutions have atoms that replace the solvent atoms in the crystal structure:
True
Amorphous materials are:
Non-crystalline and transparent
Which of the following materials may form crystalline solids?
Polymers, Metals, and Ceramics
Atomic radius of a metal with the BCC structure is R, what is the volume of the unit cell in terms of R?
\left(\frac{\left(4R\right)}{\sqrt3}\right)^3
HCP Crystals have detectable diffraction:
never
For X-Ray diffraction, the incoming wavelength must be greater than the atomic spacing to diffract.
False
Isotropic crystals are:
Single crystals randomly oriented, Crystals with non-directional properties
Secondary Bonds are directional, and therefore create a dipole.
True
Fick’s first law is a steady-state equation of diffusion
True
What material classification is typically ductile, low strength, and a poor conductor?
Polymer
What is the predominate type of bonding for sodium (Na)?
ionic
Materials with high bond energies typically have low melting temperatures.
False
Metals and ceramics are ___ while glasses are ____:
Crystalline and non-crystalline
An element with the electron configuration

has how many outermost electrons?
8
Atoms near the ____ of the periodic table are considered more electronegative:
right
Metallic bonds are directional, and therefore create a dipole:
False
What material classification is typically brittle, high strength, highly crystalline, and a poor conductor?
Ceramic
What is the predominant type of bonding for carbon (C)?
Covalent
What is the Atomic Packing Factor (APF)?
Number of equivalent atoms within a unit cell
Van der Waals bonds undergo:
Secondary attraction of atomic dipoles
When two atoms are at an equilibrium distance for bonding, the sum of the repulsive and attractive energies equals:
Trough negative energy (slope = 0) vs. r
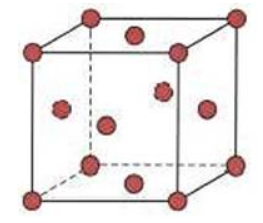
The drawing below represents the unit cell for which crystal?
FCC
How many atoms are associated with an FCC unit cell?
4
What is the coordination number of an atom in an HCP configuration?
12
What is the coordination number of an atom in an BCC configuration?
8
What is the coordination number of an atom in an FCC configuration?
12
How many atoms are associated with an BCC unit cell?
2
How many atoms are associated with an HCP unit cell?
6
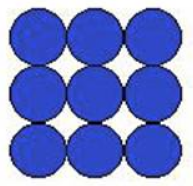
Below is a plane through a simple-cubic crystal structure. What set of indices below represents this structure?
(100)
Metal with a face-centered cubic structure, calculate the atomic radius in terms of the unit cell dimension (a).
a\left(\frac{\sqrt2}{4}\right)
Predominately covalent bonds undergo:
Electron sharing from one atom to the bonded atoms(s)
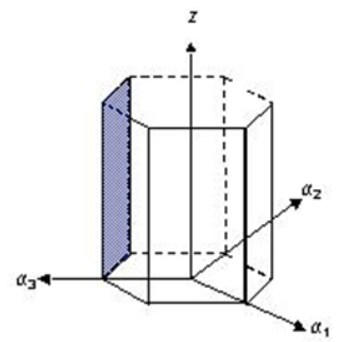
What are the Miller indices for the plane shown below?
(1̅ 0 1 0)
Completely destructive interference is detectable with X-ray diffraction detectors
False
Substitutional solid solutions have atoms within the spacing between the crystal structures
False
Increased temperature decreases the number of vacancies in a crystal structure
False
Diffusion coefficient decreases with increasing temperature
False
Material temperature is a measure of atomic vibration frequency and amplitude
True
High-angle grain boundaries typically have atoms with a higher energy than low- angle grain boundaries
True
Diffusion mechanisms include:
Vacancy Diffusion, Interstitial Diffusion
Interstitial solid solutions have atoms within the spacing between the crystal sturctures
True
For a "solid solution," the solute is
the atoms of lesser concentration
FCC crystals have detectable diffraction:
if h, k, l are all even or all odd
Point defects include:
atomic substitutions, interstitial spacing, vacancies
For a "solid solution," the solvent is:
the host, the atoms of highest concentration
Planar defects DO NOT include:
interstitial defects