Innovations in Degradable Polymers
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
why aren’t most research articles pursued by industry?
not economical
takes many years to implement on industrial scale
what does catalytic chemical recycling of post-consumer polyethylene start with?
post-consumer water jug (shred it)
steps of catalytic chemical recycling of post-consumer polyethylene
react shredded water drug w dehydrogenation catalyst → 0.5% of C-C backbone bonds dehydrogenated to C=C w/o change in MW
cross-metathesis w 2-hydroxyethyl acrylate → MW goes down (chains breaking)
hydrogenation of C=C bonds
transesterification with diethanolamine (8% reacts)
repolymerization w MW = 80kDa → tensile strength now similar to og HDPE
depolymerization w ethylene glycol and triazabicyclodecene (TBD) → once recycled to monomer, can be repolymerized
step 1 of catalytic chemical recycling of post-consumer polyethylene
react shredded water drug with dehydrogenation catalyst
approximately 0.5% of C-C backbone bonds dehydrogenated to C=C double bonds without a change in MW
essentially installing double bonds/unsaturated units without breaking polymer
step 2 of catalytic chemical recycling of post-consumer polyethylene
cross-metathesis with 2-hydroxyethyl acrylate (cheap)
essentially switch partners of double bond, adding new endgroup (breaking polymer chains in the process)
results in ethylene byproduct
end group = hydroxymethyl methyl methacrylate
MW goes down quite a bit due to chains breaking
step 3 of catalytic chemical recycling of post-consumer polyethylene
hydrogenztion of C=C bonds
now only C-C single bonds
repolymerization after this step made similar polymer Mn but much smaller Mw
→ brittle & poor tensile behavior bc of lower Mw
step 4 of catalytic chemical recycling of post-consumer polyethylene
transesterification with diethanolamine (8% of hydrogenated material reacts)
step 5 of catalytic chemical recycling of post-consumer polyethylene
repolymerization with Mw - 80 kDa
tensile strength now similar to original HDPE
step 6 of catalytic chemical recycling of post-consumer polyethylene
depolymerization with ethylene glycol and triazabicyclodecene (TBD)
once chemical is recycled to monomer, can be repolymerized (circular)
cross metathesis
type of olefin metathesis rxn that involves the exchange of substituents b/w different olefins thru the breaking & reforming of C=C bonds
catalyzed by metal complexes
transesterification
process of exchanging organic functional group of an ester with an alcohol
which new research innovation is a bottom-up approach?
chemically
bottom-up approach
synthesize monomer first and then polymerize/recycle it (start with a chemical reaction)
starting point for Chemically Recyclable Linear and Branched Polyethylenes
lactone
a cyclic compound with an ester and an internal double bond
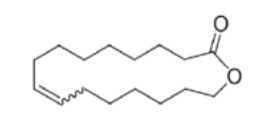
steps of Chemically Recyclable Linear and Branched Polyethylenes
ring open with methanol reflux & Sc catalyst (lewis acid accepts e- pair)
ring-opening metathesis polymerization w cyclooctenehydrogenate
Hydrogenation → yields telechelic monomers (2 different reactive end groups)
Transesterification for polymerization → no matter size of starting monomers, high MW polyethylene obtained
depolymerization by methanol reflux & Sc catalyst
what is required for branched polymerization via Chemically Recyclable Linear and Branched Polyethylenes?
trifunctional monomer
step 1 of Chemically Recyclable Linear and Branched Polyethylenes
ring open w methanol reflux & Sc catalyst
Sc catalyst = lewis acid that accepts an electon pair, making C more electrophilic
step 2 of Chemically Recyclable Linear and Branched Polyethylenes
ring opening metathesis polymerization with cyclooctene
step 3 of Chemically Recyclable Linear and Branched Polyethylenes
hydrogenation
Mn was selected in the range b/w 2-16 kDa
yields telechelic monomers (2 diff reactive end groups)
A reacts w B on another monomer
telechelic monomers
type of polymer that has reactive groups located at both ends of linear structure
step 4 of Chemically Recyclable Linear and Branched Polyethylenes
transesterification for polymerization
no matter size of starting monomers, high MW polyethylene obtained (~100kDa)
step 5 of Chemically Recyclable Linear and Branched Polyethylenes
depolymerization by methanol reflux & Sc catalyst
can do branched polymerization with a trifunctional monomer
which approach starts with a system known to be easily chemically recycled?
Chemically Recyclable Thermoplastics from Cyclic Acetals
what polymer is known to be easily chemically recycled and used in Chemically Recyclable Thermoplastics from Cyclic Acetals?
poly(acetals)
poly(acetals)
easily recycled to monomer using trace acid and heat (<150C)
liquid
easily distilled off in high purity
in the past, MW weren’t high enough to make useful materials
high MW version has good tensile strength
the high MW version of poly(acetals) has good _______ strength
tensile
pros of poly(1,3-dioxoiane) - a chemically recyclable thermoplastic
tough thermoplastic
thermally stable to 325 C
monomer recovered in 98% yeield
synthesized from inexpensive monomer w bio-sourcing options
rapidly polymerized
describe use of RD-CROP of cyclic acetals
start with dormant species with X group
MOMBr adds Br to end of growing polymer chain forming active species
Br stabilizes and allows high MW to be reached with high control
monomer addition
cycles back to dormant species
the stress-strain curve for PDXL is very similar to which commercial polymer?
iPP = isotactic poly(propylene)
triggered depolymerization enables…
chemical recycling to monomer
steps of triggered depolymerization → chemical recycling in Chemically Recyclable Thermoplastics from Cyclic Acetals
collect polymer
add acid, heat to 140-150
distill
recover monomer
can pure DXL be recovered from mixed waste stream?
yes
96% recovery