4.11 Basics concepts of organic chemistry
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
what is a hydrocarbon?
compound of hydrogen and carbon only
what is crude oil?
mixture of lots of chemicals, some of which are hydrocarbons
what is formula for alkanes?
Cn H2n+2
why are alkanes described as saturated?
has single bonds only
why are alkanes described as aliphatic?
hydrocarbons joined together in straight chains, branches chains or non-aromatic rings
what is the ending of side chain? e.g. butane?
-yl
butyl
what are cycloalkanes?
rings named after the number of carbons in the ring
what are structural isomers?
compounds with same molecular formula but with different structural formula
what is the prefix for 2 same function group?
di-
what is the prefix for 3 same functional group?
tri-
Structural isomer have similar ______ properties but different ________ properties
chemical
structural
what is a functional group?
a group of atoms responsible for characteristic reactions of a compound
what shows that a compound is an alkene?
contains a carbon-carbon double bond
what shows that a compound is an alcohol?
contains hydroxyl (OH) group
why are alkenes unsaturated?
has carbon-carbon double bonds
how do you name alkenes?
number of carbon atoms of longest chain
find the name of that chain
count along to start of double bond
alkene uses suffix -ene
2 double bonds is -diene
3 double bonds is -triene
the middle of main chain adds an ‘a’ for -di or -tri
any side chains are named at start of the name
what is an aromatic compound?
a compound containing a benzene ring
Draw a picture of benzene
sometimes circle representing the delocalisation
or lines showing double bonds
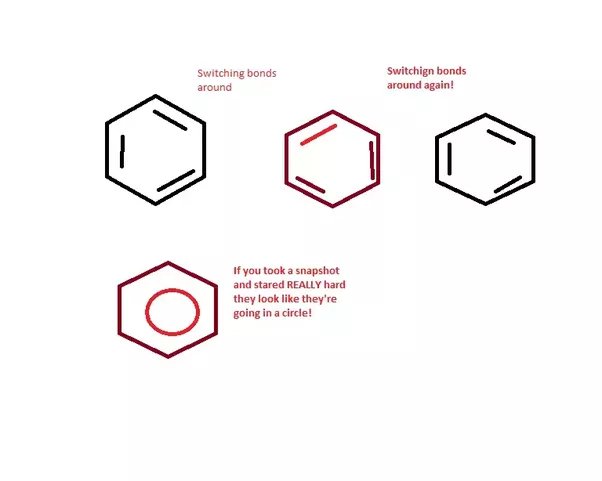
what is benzene described as structure wise?
cyclic arene
aromatic compound
why can in benzene the double bonds move around the ring?
electrons in double bonds are delocalised so can move around
what are the features of alcohols?
family of compounds containing functional group -OH
name of alchold’s end with -ol
can be structural isomer of the functional group
when naming does side chains or alcohol group take priority?
alcohol group
what can alcohols be classified?
primary
secondary
tertiary
how do alcohols be classified into primary, secondary and tertiary?
depending on which carbon atom the -OH is attached to
how would a primary alcohol looks like?
the carbon with -OH is attached to one alkyl group
how would a secondary alcohol look like?
the carbon with -OH is attached to 2 alkyl group
how would a tertiary alcohol look like?
the carbon with -OH is attached to 3 alkyl group
why are sigma bonds formed?
orbitals overlap
can sigma bonds rotate?
yes
where are sigma bonds found?
between different bonded atoms
why are pie bonds formed?
2 orbitals overlap sideways
can pie bonds rotate?
no
how many pie and sigma bonds in one single bond?
one sigma bond
no pie bond
how many pie and sigma bonds in double bond?
one pie bond
one sigma bond
how many pie and sigma bonds in a triple bond?
one pie bond
two sigma bonds