KEY TO DECIPHERING CHEMICAL REACTIONS
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Formulas of chemicals to the left of arrow
Chemicals known as reactants - what chemicals were present before the reaction occurred
Formulas of chemicals to the right of arrow
Chemicals known as the products - what chemicals were present after the reaction occurred
→
“Yields”, or “react to form;” indicates the result of a reaction
↔
Used in place of a single arrow to indicate that a reaction is reversible; that the products can react to reform the reactants
( l )
A reactant or product in liquid state
(aq)
A reactant or product in an aqueous state (dissolved in water)
(s)
A reactant or product in the solid state; also used to indicate a precipitate
(g)
A reactant or product in the gaseous state
↑
Alternative to (g), but used only to indicate a gaseous product
↓
Alternative to (s), but used only to indicate a precipitate
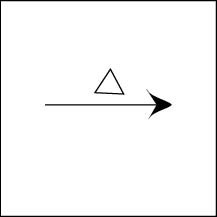
Reactants are heated

Temperature at which reaction is carried out
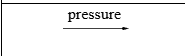
Pressure at which reaction is carried out exceeds normal atmospheric pressure
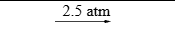
Pressure at which reaction is carried out
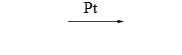
Formula of a catalyst, used to alter the rate of the reaction
Br2 H2 O2 N2 Cl2 I2 F2
Diatomic Elements