Nucleophilic Reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Nucleophile
Region of high electron density
This can be a lone pair / negative charge
Electrophile
Region of low electron density
This can be next to an electronegative atom or cation
What does a curly arrow represent?
The movement of a pair of electrons
What does SN mean?
Substitution nucleophilic
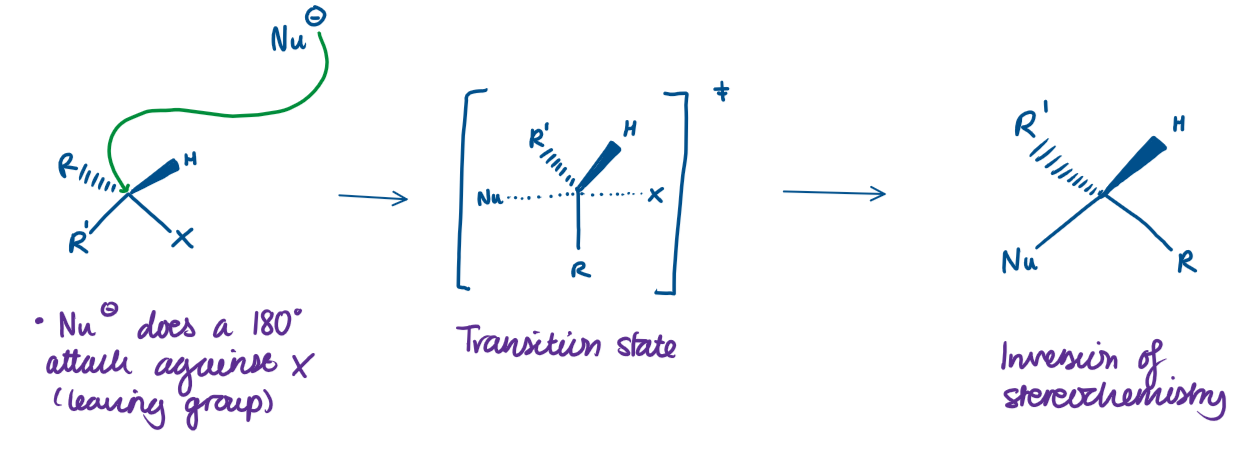
What is SN2?
Substitution Nucleophilic Bimolecular
One step reaction (all at once)
The nucleophile attacks the carbon at the same time the leaving group leaves
The rate depends on both the concentration of the substrate (the molecule being attacked), and the concentration of the nucleophile
Example of an SN2 reaction
CH3Br + OH- → CH3OH + Br-
OH- is the nucleophile and it attacks
Br- is the leaving group and leaves
Key points of an SN2 reaction:
Happens in 1 step
Strong nucleophile needed
Works best with primary carbons
Inversion of configuration (the molecule flips like an umbrella)
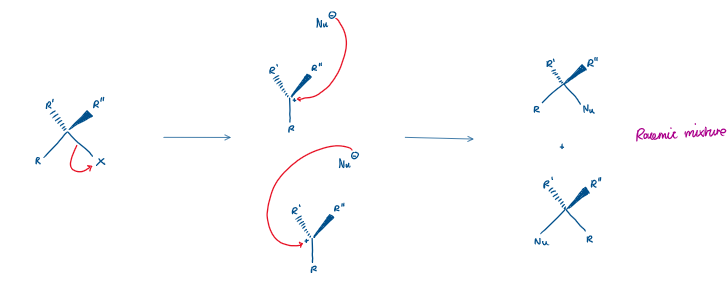
What is SN1?
Substitution Nucleophilic Unimolecular
Two step reaction (one at a time)
Leaving group leaves first, forming a carbocation (C+)
Nucleophile attacks the carbocation
The formation of the carbocation is the slow step, so it is the rate determining step
Therefore the rate depends only on substrate concentration
Example of SN1:
(CH3)3CBr --> (CH3)3C+ + Br-
(CH3)3C+ + H2O --> (CH3)3COH + H+
Key points of SN1:
Happens in 2 steps
Carbocation intermediate
Works best with tertiary carbons
Weak nucleophile is okay
Causes racemization (the mix of both enantiomers, no inversion)
What is steric hindrance?
The crowding around a molecule’s reactive site makes it hard for another molecule (like a nucleophile) to get close enough to react.
Imagine a nucleophile (like OH⁻) trying to attack the carbon where Br is attached:
In methyl or primary cases, it’s easy — not much in the way.
In tertiary cases, all those CH₃ groups are bulky and block access to the carbon.
That’s steric hindrance — the bulky groups literally get in the way.
Steric hindrance in SN1?
The leaving group leaves first, forming a carbocation, and the nucleophile attacks after when there’s more space
What is E1 and E2?
The elimination versions of SN1 and SN2.
E = Elimination
Instead of a nucleophile replacing the leaving group (like in SN1 and SN2), the base removes a hydrogen atom and the leaving group leaves, forming a double bond
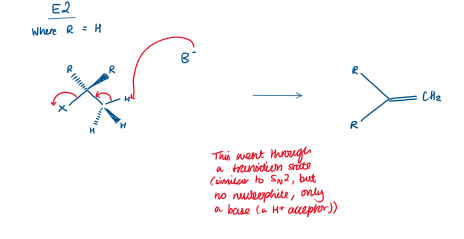
E2 reaction
So three things happen simultaneously:
The base takes a hydrogen.
The C–H bond breaks.
The C–X (leaving group) bond breaks and a C=C double bond forms.
E1 reaction
2 step reaction like SN1
Leaving group leaves first, forming a carbocation.
A base removes a proton (H⁺) from a β-carbon → double bond forms.
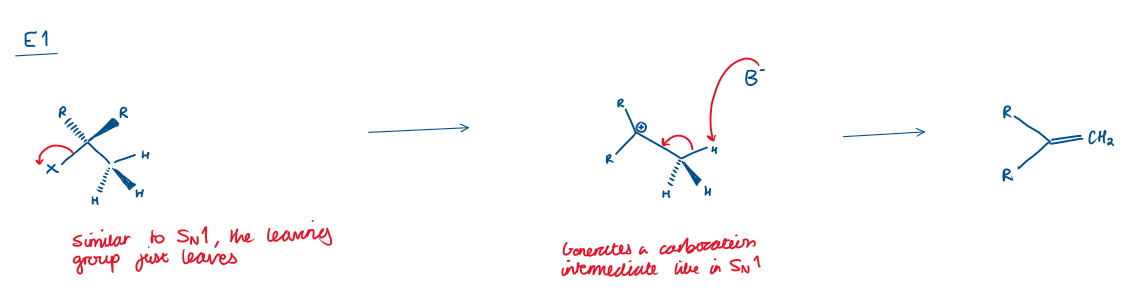
Key features of E1:
Two step reaction
Carbocation intermediate
Weak base
Tertiary carbocations
In E1, what does R =
Aryl alkyl
In E2, what does R =
H