2: Peripheral Cholinergic Neurotransmission
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
Agents indirectly modulating NMJ transmission
-do not act directly on the Nm channels but indirectly modulate the amount or the life-time of ACh released within the junctional cleft
Naturally-occurring agents affecting ACh release
-Botulinum toxins (BoTXs/BoNTs)
-β-bungarotoxin (β-BuTx)
-α-latrotoxin (α-LTX)
-Tetanus neurotoxin (TeNT)
Botulinum toxins (BoTXs/BoNTs)
-toxins are produced by Clostridium botulinum – a Gram-positive bacterium
-responsible for botulism that may happen through food poisoning, colonisation of the infant gut or via exposed wounds
-preferentially target the motor nerve terminal releasing ACh at the NMJs
How does botulinum toxins work?
-Selectively cleave SNAREs via C termini protease activity
-Heavy chains bind to some gangliosides specific to the motor neurons- allows them to be endocytosed specifically into these nerves
-There are seven distinct BoTX isoforms and they target specific SNARE proteins within the motor neuron
How is botulinum toxin administered?
-Injected locally
-Botulinum toxin type A (BoTX-A) is widely used for cosmetic purposes as BOTOX therapy for removing facial lines and wrinkles.
-Can be used in treatment of certain ophthalmic conditions associated with spasms of eye muscles (e.g., blepharospasm).
What is botulism?
Rare, but serious and potentially fatal illness caused by a toxin produced by Clostridium botulinum bacteria.
Toxin attacks the nervous system, leading to paralysis that typically begins with drooping eyelids and can spread to the muscles controlling breathing, causing respiratory failure if untreated
β-bungarotoxin (β-BuTx)
-Present in the venom of the banded kraits
How does β-bungarotoxin (β-BuTx) work?
-Has complex mechanism of action at the NMJ involving multiple targets.
-Has phospholipase A2 activity, which causes irreversible damage to membrane phospholipids in motor nerve terminals (especially the active zone).
-Ultimately impairs ACh release.
α-latrotoxin (α-LTX)
-Agents promoting ACh release
-This neurotoxin is found in the venom of the female black widow spider
-It induces a profound paralysis by causing massive release (without even requiring any depolarisation of the motor neuron) and subsequent depletion of ACh at the NMJ.
How does α-latrotoxin (α-LTX) work?
-Mechanism of action remains still poorly understood but recent structural studies have suggested that this toxin is likely form a tetrameric Ca2+- permeable ion channel in presynaptic nerve membrane.
Tetanus neurotoxin (TeNT)
-Agents (indirectly) promoting ACh release
-produced by Clostridium tetani (a Gram-positive bacterium)
How does Tetanus neurotoxin (TeNT) work?
-enters (through endocytosis) into the motor neurons at the NMJs
-Moves towards the cell body of the motor neurons via retrograde axonal transport
-Eventually gets released into the inter-synaptic space within the spinal cord.
- Then selectively enters into some inhibitory (glycinergic) interneurons and proteolytically cleaves synaptobrevin (a v-SNARE).
-Causing disinhibition of the motor neuron which then results in violent (tetanic) contractions of skeletal muscle
What are the 2 types of cholinesterase?
-acetylcholinesterase (AChE)
-butyrylcholinesterase (BuChE)
Difference between AChE and BuChE?
While AChE is quite specific for ACh, BuChE has much broader substrate specificity - it also breaks down some local anaesthetics and suxamethonium
Where is AChE and BuChE mainly found?
-BuChE is made in the liver and found mainly in the plasma.
-AChE is found both within the presynaptic nerve ending (as a soluble form) and in the synaptic cleft/junctional folds.
What is the structure of AChE?
AChE oligomers are linked to a long collagenous tail that tethers them to the postsynaptic/postjunctional target membrane. It is this membrane-bound oligomeric form of AChE which is primarily responsible for hydrolysing ACh and thus terminating its action.
Cholinesterases
-Both AChE and BuChE are serine hydrolases.
-The hydroxyl (-OH) group of a critical serine (Ser) in these enzymes acts as a nucleophile (= a chemical species that avidly seeks for positivelycharged/partially positively-charged centres) to form initial interaction with the substrates.
Mechanism of action of cholinesterases
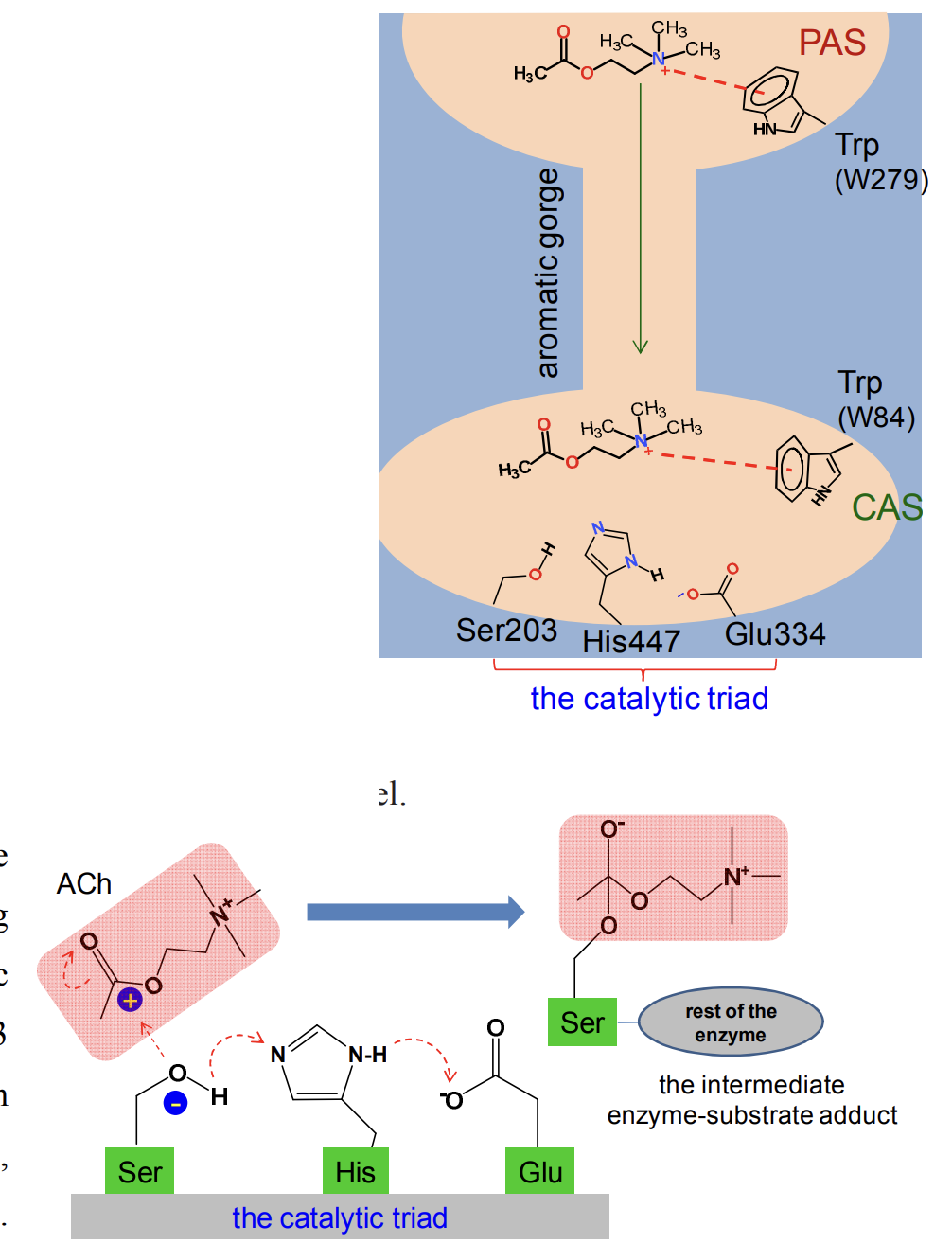
-The catalytic site lies at the base of a deep and narrow tunnel named the aromatic gorge.
-At the entrance to this gorge, there is a peripheral anionic site (PAS) where ACh is initially recognised as the substrate.
-One of the key interactions here is a cation- interaction between a specific tryptophan residue (W279 for mammalian AChE) and the charged nitrogen of ACh.
-After being ‘captured’ this way, ACh is then rapidly pushed down the gorge to the active site, primarily through cation- interactions with >10 conserved aromatic residues lining the tunnel.
-After reaching the base of the gorge, ACh binds to the catalytic anionic site (CAS) for proper positioning against the catalytic triad consisting of specific glutamate, histidine and serine (Glu334-His447-Ser203 for human AChE).
-These three residues are arranged in such a way that the -OH group of the serine loses H, turning O into electron rich or a nucleophile (-Ser-O- ).
-This allows covalent bonding with the acetyl moiety of ACh to form an intermediary acetyl-enzyme conjugate.
-From this intermediate, choline is released and remaining acetate-Ser (enzyme) bond is spontaneously hydrolysed to release acetate and regenerate the enzyme.
-This enzymatic reaction is one of the fastest and most efficient ones known in biology
Anticholinesterases (anti-ChEs)
-Bind to and inhibit AChE, thereby increasing the lifetime of the released ACh within the synaptic/junctional cleft.
-More 'intact' ACh molecules are now available for activating the cholinergic receptors at the target membranes.
-Anti-ChEs are also known as indirectly acting cholinergic agonists, given they do not activate cholinergic receptors directly
There are several ways to classify the anticholinesterases:
-Based on mechanism of inhibition
-Based on duration of action:
Classification based on mechanism of inhibition
-Reversible:
a) non-covalent (e.g. edrophonium)
b) covalent inhibitors (drugs with suffix ‘stigmine’ such as neostigmine)
-Irreversible (organophosphates, nerve gases etc.)
Classification based on duration of action
a) Short acting (edrophonium)
b) Intermediate acting (the ‘stigmines’)
c) Long acting (the organophosphates)
Edrophonium
-Reversible & short acting anti-ChEs
-The prototype of this class, interacting only to the catalytic anionic site (CAS) of the AChE enzyme.
-The interaction is readily reversible and accounts for the short duration of action (2-10 min).
-It is used to diagnose (the tensilon test) myasthenia gravis.
-Patients (humans, pets) show characteristic temporary improvement in the facial weakness and ptosis within 5-10min of receiving edrophonium injection.
stigmine
-Reversible & medium acting anti-ChEs.
-Esters of carbamic acid (instead of acetic acid) and have the suffix 'stigmine'.
-All except physostigmine and rivastigmine are charged and therefore act only peripherally.
-With their basic group, they interact with the CAS site of AChE and transfer carbamyl group to the hydroxy group of Ser 203.
-Carbamylated AChE is more stable than acetylated AChE and takes minutes to hydrolyze.
Neostigmine
-Reversible & medium acting anti-ChEs
-Used intravenously to reverse neuromuscular blockade after surgery and orally to treat myasthenia gravis.
-In Alzheimer's disease (AD), there is significant reduction in cholinergic neurotransmission in the basal forebrain. Few brain permeable anticholinesterases are approved for Alzheimer’s patients to improve some symptoms.
Organophosphorous compounds
-Irreversible & long acting anti-ChEs
-containing a pentavalent phosphorous atom connected to three oxygen atoms along with a labile group, such as fluoride or an organic group.
-The labile group can be released and the Ser 203 hydroxyl group is then phosphorylated.
-phosphorylated enzyme complex is extremely stable and dissociates with a half-life of hundreds of hours.
-The complex can undergo 'aging' – a process in which oxygen– phosphorus bonds of these agents undergo spontaneous rearrangement to form even a tighter complex with AChE.
-Thus, organophosphate inhibition is essentially irreversible, and the body must synthesize new AChE proteins to restore AChE activity
Clinical use of organophosphates
-Clinical use of these agents is limited.
-Many of them are used as insecticides.
-This group of compounds includes some notorious chemical warfare agents - the "nerve gases" which readily penetrate the skin and cause both acute and chronic toxic effects.
Acute poisoning by irreversible anti-AChEs
-Results in excessive salivation, severe bradycardia, hypotension and difficulty in breathing.
-Standard treatment involves the use of atropine in large doses which can reach the brain in sufficient concentrations to suppress the central and peripheral cholinergic symptoms associated with the poisoning.
Pralidoxime
-Atropine is virtually without effect against the peripheral neuromuscular compromise which can be reversed by pralidoxime (2-PAM), a AChE reactivator.
-The cationic group of pralidoxime interacts with the anionic site of AChE, which brings the oxime group into close proximity with the phosphorylated serine.
-The oxime group is a very strong nucleophile and lures the phosphate group away from the Ser hydroxyl of the enzyme.
-The effectiveness of AChE reactivation is however limited to within a few hours of exposure as the phosphorylated AChE undergoes the 'aging' that renders the phosphorylated group no longer being susceptible to nucleophilic attack.
What are the four different changes in membrane potential in response to stimulation of the preganglionic nerve?
a) an initial EPSP (via Nn receptors) that may result in an action potential
b) an IPSP mediated by muscarinic M2 receptors (Gi/o-coupled)
c) a secondary slow EPSP mediated by muscarinic M1 receptors (Gq/11-coupled)
d) a late, slow EPSP mediated by various neuropeptides.
What are the limitations of agents acting at the ganglia?
-Could either stimulate or block ganglionic transmission but they suffer from limited therapeutic uses due to lack of selectivity between sympathetic and parasympathetic ganglia
-Few can also have effects on the CNS.
Ganglionic stimulants
-Nn receptor agonists: this group consists of nicotine and related molecules that bind to and stimulate the Nn receptors at the ganglia. If used in higher concentration, they also cause ganglionic blockade.
-Nicotine is a tertiary amine found in tobacco leaves.
-In low concentration, nicotine stimulates Nn channels whilst at higher doses, it desensitizes the channel (typical for LGICs).
Tetramethylammonium (TMA)
-ganglionic stimulant
-only stimulates ganglia but doesn’t block it
Ganglionic blocking agents
There are two categories of agents:
-The prototype of the first group - nicotine, initially stimulates the ganglia as an agonist and then blocks them by causing a persistent depolarization. - Compounds in the second category impair transmission either by competing with ACh for binding at ganglionic Nn receptors or by blocking the channel pores (hexamethonium).
-Their mechanism of action is analogous to that of the non-depolarising type blockers at the NMJs.
tubucurarine
Competitive antagonist
hexamethonium
Blocking the channel pores for nACh
Clinical uses of ganglionic blocking agents
-The earliest anti-hypertensive agents but all are clinically obsolete by now due to numerous side effects, largely coming from their inability to discriminate between sympathetic and parasympathetic ganglia as well as between the ANS and CNS for some agents.
mAChRs
-Present in autonomic ganglia and on some cells (e.g., vascular endothelial cells) that receive little or no cholinergic innervations.
-Of the 5 different mAChR subtypes, M1, M2 and M3 are the most important in the periphery.
-In majority of the cases, at least two mAChR subtypes (typically M3 and either M1/M2) are present in the autonomic effector tissues and both contribute to the observed tissue response to ACh and/or cholinergic agonists.
-Muscarinic antagonists have greater clinical uses than the agonists
Agonists of mAChRs
-Directly-acting parasympathomimetic or cholinomimetic agents.
-mAChR agonists can be broadly classified into two groups:
1) choline esters, which include ACh and synthetic esters of choline
2) some naturally occurring cholinomimetic alkaloids (and their derivatives)
To be useful for therapeutic benefits, they should ideally have:
a) greater stability (and thus longer duration of action) than ACh
b) selectivity for mAChRs over nAChRs
c) selectivity for specific mAChR subtype (limited success so far, work in progress)
d) being restricted to the periphery (for relevant peripheral diseases)
carbachol
synthetic esters of choline (popular as an experimental compound)
Antagonists of mAChRs
-These are directly acting anti-cholinergic or parasympatholytic agents.
-Competitive antagonists, block ACh binding to its orthosteric binding site on mAChRs present on effector cells at parasympathetic neuro-effector junctions. They also block the few exceptional sympathetic neurons that are cholinergic.
-In general, they cause little blockade of nAChRs and thus have little or no action at NMJs or autonomic ganglia.
anti-muscarinic receptor agents
-contain an ester and basic groups like that of ACh but bulky aromatic group replacing the acetyl moiety
-atropine
-ipratropium
-tropicamide
-cyclopentolate
-solifenacin
atropine
naturally occurring alkaloids anti-muscarinic
ipratropium
semi-synthetic derivatives anti-muscarinic
tropicamide, cyclopentolate, solifenacin
synthetic anti-muscarinic agents
Selective for specific mAChR subtypes
-pirenzepine is M1 selective
-darifenacin and solifenacin are M3 selective
Eye
-autonomic nervous system influences pupillary diameter for light adaptation, curvature of the lens for adjustment of the focal length and drainage of aqueous humour
-smooth muscle (constrictor/sphincter muscle) of the iris and the ciliary muscle contain muscarinic M3 receptors (predominantly) and are innervated by parasympathetic cholinergic nerves
pilocarpine
-naturally occurring cholinomimetic alkaloids
-direct stimulation of M3 receptors makes the sphincter muscle contract, producing miosis.
-This together with contraction of ciliary muscles aids drainage of aqueous humour and thus reducing intra-ocular pressure (IOP) in glaucoma.
-used to promote salivary secretion in xerostomia (a dry mouth problem, most common complications associated with radiotherapy of head and neck cancer). It is also used to promote salivary and lacrimal secretions in Sjögren’s syndrome which is an autoimmune disease of glands.
-similar uses in veterinary medical practice - to manage particular dry eye problem (Keratoconjunctivitis sicca) for dogs.
mAChR antagonists effects on M3
-Used topically to block those M3 receptors and produce pupillary dilatation (mydriasis) and paralysis of the ciliary muscle (cycloplegia)
–both of these changes help in ophthalmic examinations (e.g. funduscopy).
-Short-acting, relatively weak agents, such as tropicamide (action lasts for 4–6 hours) and cyclopentolate (action up to 24 hours) are typically preferred over atropine (action may last up to 7 days).
Heart
Parasympathetic (vagus) stimulation or application of muscarinic agonists, via muscarinic M2 receptor, will reduce the frequency of heart rate (negative chronotropic effect)
Circulation
-Blood vessels largely lack parasympathetic innervation, but ACh/ muscarinic agonists dilate arteries in the coronary circulation and in skeletal muscle vascular beds via activation of endothelial muscarinic (mainly M3) receptors.
-The mAChR antagonists are used in a variety of clinical situations for both human and animals, predominantly to inhibit effects of parasympathetic activity in the respiratory tract, eye, urinary tract, GI tract and heart.
Urinary Bladder
-M3 selective antagonists such as solifenacin can be used in the treatment of urinary incontinence through their selective inhibition of M3 receptors that mediate contraction of the detrusor muscle in the urinary bladder.
Airways
-several mAChRs are present in nerves supplied to the airways.
-ACh released by parasympathetic nerves upon stimulation, acts directly at M3 receptors on the airway smooth muscle to cause bronchoconstriction. Activation of these receptors at the lung epithelia also promotes mucous secretion. This happens normally as a 'vagal tone' but many irritants (dust, allergens, histamine, some gases etc.) via local sensory nerves can make it more prominent in the form of a 'vagally-mediated reflex bronchospasm'.
-In COPD but unlike in asthma, this vagal tone seems to be the major reversible brochoconstrictive component. Blocking the M3 receptors in bronchial smooth muscles results in a decrease in smooth muscle tone causing bronchodilation. The effect is small in normal airways but significant in airways from COPD patients.
-Medications include short-acting muscarinic antagonists (SAMAs) such as ipratropium, and long-acting muscarinic antagonists (LAMA) such as tiotropium and glycopyrronium.
-All have higher selectivity for M3 receptors than for M2 receptors, and the long duration of LAMAs stems from their slow dissociation from the M3 receptors whilst they dissociate from M2 receptors much faster. LAMAs can also be effective as additional bronchodilators if asthma in patient is not adequately controlled by -adrenoceptor stimulants
Various other secretions:
Some muscarinic antagonists (e.g. glycopyrronium) that do not cross the BBB, can be used to reduce excessive salivation ('drooling', either drug induced or associated with heavy-metal poisoning and Parkinson's disease).
Atropine in large doses is used as the standard treatment of poisoning by irreversible, long-acting anti-cholinesterases such as organophosphates.