GENET 390: Topic 1 - DNA structure + Function
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
Where are the bases located for DNA? What issue does this cause?
Within the helix
Info = hidden from what can read it = HARD TO ACCESS
What is the structure of DNA? How was this determined + what was the OG hypothesis of how DNA looked? Why was the OG hypothesis what it was?
DNA = Antiparallel double helix with bases pointing in
Conclusion from x-ray crystallography
OG hypothesis = 3 strand with bases outside
3 strand bc collagen = very stable + 3 strand
Bases outside bc info needs to be accessed
What is the Directionality of DNA + what does this mean about the ends of DNA?
5’ —> 3’
MEANS: end of DNA = chemically + functionally different from each other
5’ end = reactive with 3 ‘ end
Ends are never reactive within themselves
What bonds are formed during DNA polymerization + what rxn occurs to form these bonds?
Phosphodiester bonds via esterification rxns
Why are ntds considered High E molecules. What makes them High E + why?
Because they have phosphates (PO4-)
Why high E? = bc like charges repel
***What are the 3 points of Chargaff’s rule?
DNA only uses ATGC
A = T and C = G
Variation between A:T and G:C rations in different species
What are the 3 types of DNA helices?
A
B
Z
Which is the most common form of DNA? and what are all the characteristics (direction of spiral, radius diameter etc.)
B DNA
Right handed
Asymmetrical = Major + minor groove
Radius = 10 A (A = 0.1 nm)
Diameter = 19 nm
Is A DNA seen often. In what conditions is A DNA normally seen?
NO not seen often
Seen in DEHYDRATION conditions
What direction is A DNA spiral?
Right handed
****What is the major difference between A DNA + B DNA
BASE PAIRING = not perfectly perpendicular to backbone (unlike B DNA)
How does the alignment of the base pairs affect the major + minor grooves of A DNA?
Major groove = DEEP + NARROW
Minor groove = Wide + Shallow
When is Z DNA seen? What conditions (in vitro vs. in vivo)
Not Normally seen TRANSIENT
In vivo = Possibly alleviate negative supercoiling
In vitro = Occurs in High salt conditions
What direction is the Z DNA spiral?
Left handed
structure repeats every 2 bp
*****What are the 4 main DNA stabilizing forces?
Covalent (phosphodiester)
H - bonds
Base stacking interactions
Shell of hydration
***Of the 4 stabilizing forces which is the main force in ssDNA?
Covalent bonds
****What does the Covalent force provide? (2 things)
Structure
Stability
Are the Phosphodiester covalent bonds Specific?
NO
Will join any ntd in any order
****Which of the stabilizing forces the most if not all the SPECIFICTY?
H-bonds (base pairing)
Do H-bonds contribute a lot to structural stability?
Keeps double strand together
strength in numbers
****What are the 2 components of Base stacking interactions?
Hydrophobic Interactions (bc bases are hydrophobic)
Interactions of aromatic rings
***What 2 forces/interactions are involved in AROMATIC interactions of base stacking?
Pi- Pi stacking
Van der wall
***Why is DNA a helix?
The Turn Maximizes ring stacking interactions = favorable
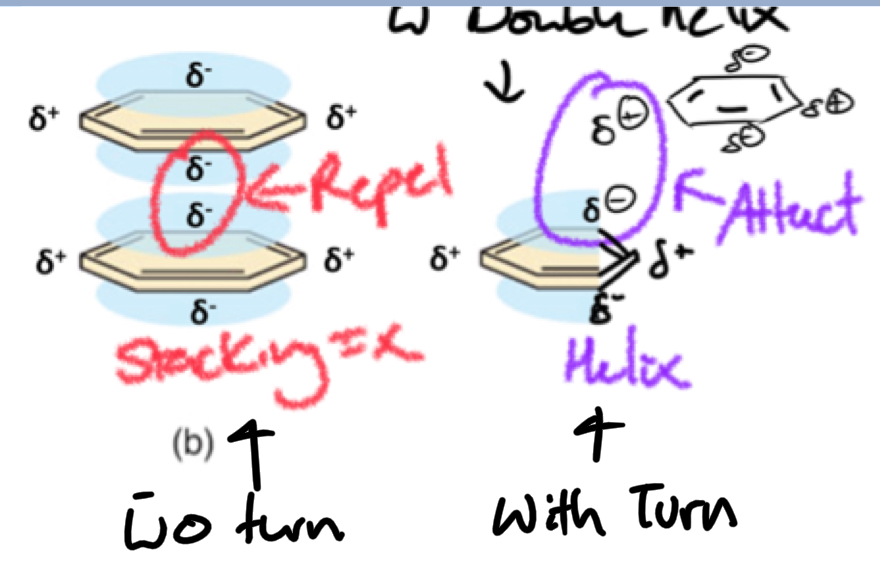
*****What is the MAIN STABILIZING FORCE of DOUBLE STRANDED HELIX?
Hydrophobic interactions of base stacking
****Are hydrophobic base stacking interactions present in ssDNA?
YES
But stacking forces = maximized in dsDNA
****What does the SHELL OF HYDRATION PROVIDE? (2 things)
Solubility
Stability
How does the Shell of hydration provide solubility?
H2O interacts with PO4- = Increase solubility
*****How does shell of Hydration Provide stability?
PO4- can be in REPUSION with one another
Shell of hydration contains COUNTER IONS = decrease repulsion
****Which counter ION is especially favorable for decreasing repulsion?
Mg2+
increases stability of DNA
****Why does DNA form ds Duplexes by Watson-crick bp whenever possible?
Nucleic acids/bases = Slightly Hydrophobic = exclude water whenever possible
Does DNA have a Charge?
YES
the BACKBONE is uniformly NEGATIVELY charged
NON SEQUENCE SPECIFIC
***Is DNA reactive?
YES
lots of places for spontaneous chemical modifications
*****What are 3 spontaneous chemical mods that DNA can experience?
Acid induced DEPURINATION
Hydrolytic DEAMINATION
Spontaneous HYDROLYSIS of backbone
*****What is Acid induced depurination?
Weak point in DNA = Glycosidic bond (join base to sugar)
Acid catalyzed break of Glycosidic bond
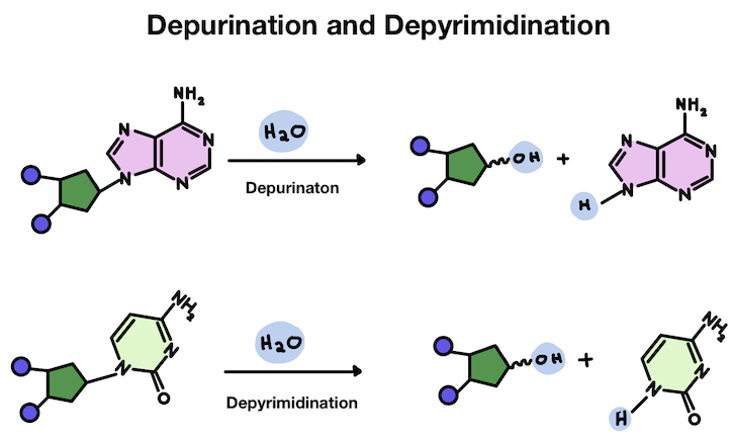
*****Is depurination of depyrimidination more common? Which Nucleic base is normally lost?
Depurination = more common
A usually lost
*****What is Hydrolytic Deamination? How does it work? What occurs?
Cytosine loses NH2 (amino) ==> URACIL
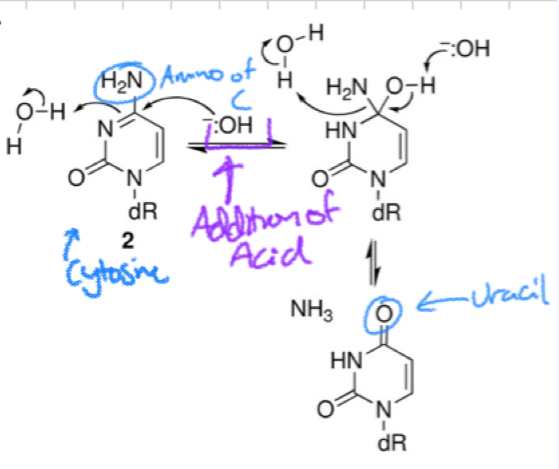
****What is a Common epigenetic Modification that affects Hydrolytic Deamination? How does it affect Deamination?
Methylation of Cytosine
= Increases speed of Deamination
Define epigenetic modification
Mod that affects gene expression without altering the DNA sequence
Info stays same but access to it changes
What are the 2 types of epigenetic modifications?
Modify DNA itself
Modify histones
Both change access to DNA
*****What is Spontaneous Hydrolysis of Backbone? What is it + how does it work?
Breaking of phosphodiester bond
very slow under physiological conditions for DNA
***True or false Spontaneous hydrolysis of back bone faster for RNA than it is for DNA? WHY?
True
2’ OH of RNA makes it more reactive and susceptible to hydrolysis
***Of the 3 possible spontaneous chemical modifications of DNA, Which is a common mutational driver/event?
Hydrolytic Deamination
C —> U
Are more proteins major groove associated or minor groove associated? Why?
Major groove
can see more DNA than minor groove
*****What are the 2 major bond types between DNA + Protein?
Non-sequence specific
Sequence specific
****How do the amino acids interact for NON SEQ. Specific?
Interact with Negative charge of Backbone
Easy with Positive charged A.A.
****How do the amino acids interact with SEQ. Specific?
A.A. Interact with Exocyclic groups
****What are Exocyclic groups?
Functional group not in ring
sticking out
****What type of bonds do A.A.’s from with Exocyclic groups?
Transient H-Bonds
transient = non-permanent
*****What are the 3 Common DNA binding motifs on proteins? What portion of the DNA do they each interact with?
Positive charged A.A. (associate with backbone)
Patterns of Polar uncharged A.A. (associate in BASE-PAIR specific way)
Compatible H-bond acceptor + Donor
*****What are the 3 main differences between RNA + DNA?
2’ OH
U vs T
Modified bases
*****What are the 5 ways The presence of 2’ OH changes RNA from DNA?
RNA = More reactive + Polar
RNA = More relaxed helix
A-Form geometry
RNA function compared to DNA
RNA doesn’t mind being single stranded
*****Why is RNA more reactive than DNA?
2’ OH
Chemical attack on 2’ OH (O = electronegative = nucleophile = reactive)
Cleavage of backbone
2’ OH might want to bind to 5’ in place of 3’ OH
*****Compare Differences in RNA vs. DNA function + identify reason for differences.
RNA = more diverse function
Bc of REACTIVITY
DNA = genetic storage
Bc of STABILITY
****Why doesn’t RNA mind being single stranded? What does this result in?
More relaxed + open helix drives single strandedness
free bases
******U vs. T. Why is T better for being an INFO molecule (DNA).
Spontaneous Deamination of Cytosine
****What is the Spontaneous Deamination of Cytosine
Removing amine group = converts CYTOSINE —> URACIL
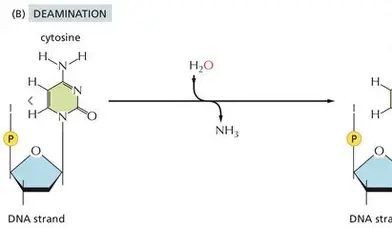
*****Why would DNA using U be an issue?
There would be an increasing the # of C —> U transition mutations (spontaneous deamination)
No way to know if the U belongs or not/mutation
If using T = will be able to tell if there was a transition mutation because U is not supposed to belong in DNA
*****Other than spontaneous deamination of C, What is another advantage that T has over U in DNA?
T = Prone to Dimers BUT they are REVERSIBLE
U dimers are NOT reversible
****True or false: U is also more prone to forming Dimers caused by UV?
False
T = more prone
****What are base modifications?
Chemical changes done to ntd bases AFTER transcription
*****How do Base modifications differ between DNA + RNA?
DNA = only C + A modified
RNA = ALL BASES MODIFIED
*****What does Base modification Aid in for RNA? + In what type of RNA is it the most common? + What type of modification is the the most common?
aids in RNA Function = Aids in GENE EXPRESSION
tRNA = most common
Methylation = most common mod
*****RNA = more reactive than DNA. What are the 3 things that it can form DUPLEXES with?
DNA
Itself eg. tRNA structure
RNA eg tRNA + rRNA
****Which is the stronger bp bond? RNA:RNA or RNA:DNA?
RNA RNA
****What are 3 examples of where RNA:DNA duplexes form?
Transcription
DNA replication (Primers = RNA)
Reverse transcription
***Why is RNA less stable than DNA outside the cell? (2 reasons)
RNases = everywhere
Weak bases (acid/base) hydrolyze RNA
****Why are RNases everywhere?
Only place RNA exists outside cell = in VIRUSES
****Explain Weak bases (acid/base) hydrolyze RNA + why it makes RNA less stable than DNA?
Spontaneous Hydrolysis of Backbone
Occurs very slowly for DNA
Faster for RNA than DNA
****True or false: Proteins read RNA better than DNA?
False
****WHY don’t proteins read RNA as well as DNA? (2 reasons)
A form = NARROW major groove
Complicated structure
****So if proteins don’t read RNA as well, What do they use to recognize RNA?
recognize 3D structure rather than sequence
RNA has to be folded in the correct structure for RNA binding proteins to recognize it
****What are the secondary structures o RNA due to?
Just bp
Intra or Inter strand H-bonding with complementary sequence
****What are the Tertiary structures of RNA due to?
3D shape due to bp of secondary structure
FOLDING due to complementary bp of secondary sequences
*****What are the 2 things that facilitate secondary + tertiary RNA structures?
RNA helicase
RNA chaperones