Lec 4 Metabolism and Cell energy
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
The reactions inThe reactions in the pathways of glycolysis and the citric acid cycle break down glucose into smaller molecules. Therefore, these pathways the pathways
Catabolic pathways
Catabolism is the breakdown of molecules into smaller units, producing ATP
What happens to bonds within chemical reactions?
During chemical reaction, atoms keep chemical identity but the bonds change
What can occur in human bloodstream due to CO2 (B)?
cells break down glucose → produce carbon dioxide.
In animals, this carbon dioxide diffuses out of cells and into the blood.
Rather than remaining dissolved in solution, most of the carbon dioxide is converted into carbonic acid.
In an aqueous (watery) environment like the ocean or the blood: carbonic acid exists as bicarbonate ions ( HCO 3 − ) and protons ( H + ) .
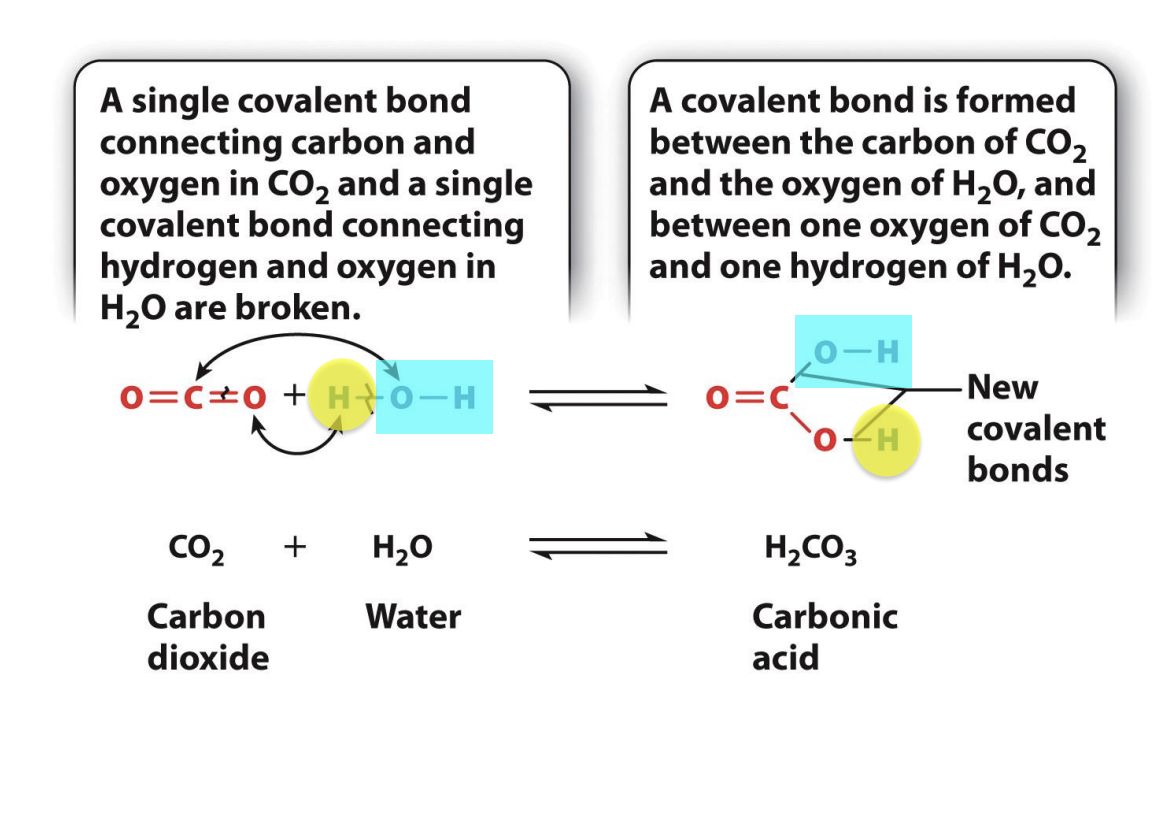
How is carbonic acid formed?
CO2 and water react to form carbonic acid
CO2 covalently bonded with double bonds
H20 covalent bonded to O with two single bonds
single covalent bond connecting Carbon and oxygen in CO2 and single covalent bond connecting hydrogen and oxygen in H20 are broken
Covalent bond is formed between carbon of CO2 and oxygen of H20, and between one oxygen of CO2 and one hydrogen of H20
Happens in Aquarius atoms -> oceans become more acidic because more CO2 dissolved in water --> increased carbonic acid
reaction is reversible
atoms stay the same, but bonds change
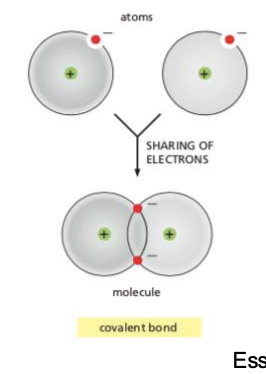
What are covalent bonds?
A chemical bond formed by a shared pair of electrons holding two different atoms together.
Covalent: sharing e-
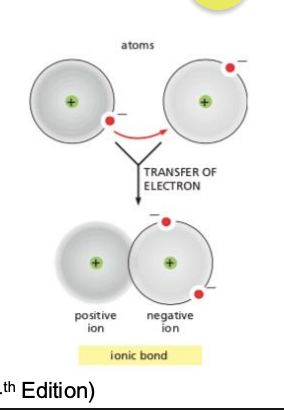
What are ionic bonds?
A chemical bond in which two ions with opposite charges associate with each other due to their difference in electronegativity.
Not shared from one atom to another: breaking and forming of ionic bonds
What kind of bond is there in the formation of carbonic acid?
Involve breaking and creating covalent bonds
What are free radicals (Reactive Oxygen Species)?
Atoms or molecules in unpaired e - --> unstable and highly reactive (need more to become stable)
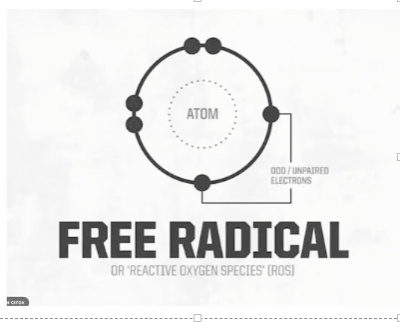
How are free radicals shown in oxygen?
Oxygen: 8 e- to be stable (not a free radical)
Loses e- is becomes unstable and wants to pick e' from the env
Common free radical in body
Single oxygen atom -> 6 (free radical)
Need to share 2 e- to be stable
b/c so much oxygen -> free radicals common in body and env
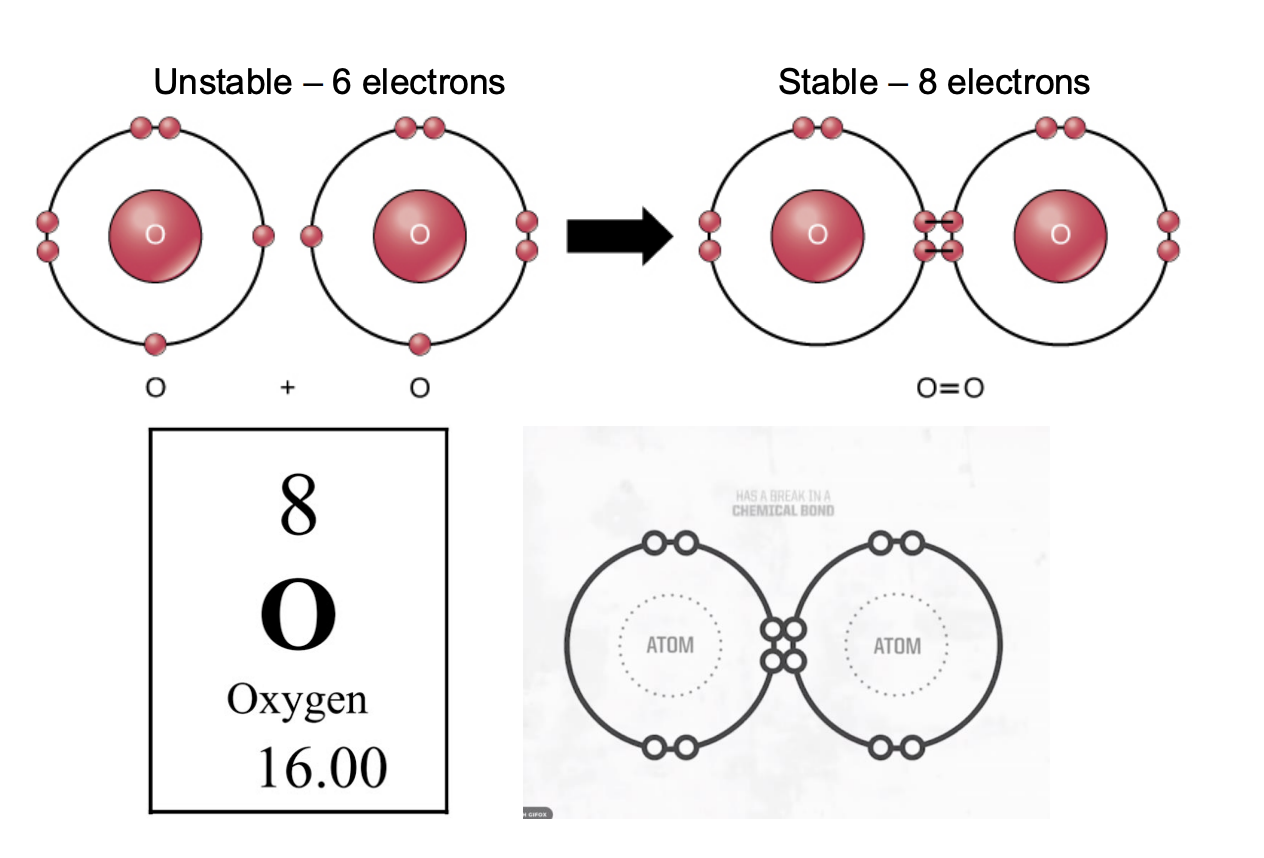
What do Reactive Oxygen species (free radicals) lead to?
Free radical steal e- from other molecules -> cause dmg to cells and tissues
Naked eyes
Apple slice oxidized (dmged from oxidation (lose of e-))
Human aging caused by free radicals
Using protection on skin and damaged by free radicals
What are antioxidants?
Antioxidants provide e- and serve as reducing agent and limits oxidation dmg to biological structures
More e- -> the more capable they are to deal with oxidation
Have a lot of e- to give

What is a popular antioxidant?
Selenium
Popular health supplement
Has a lot of e- it can give

How does selenium act as an antioxidant (slide was confusing)
Fungal species: ability to use selenium and incorporate it to produce proteins -> specialized protein (selenoprotei)
Protein can be used as an antioxidant b/c a lot of e-
Compartments: sulfuer?
Selenprotien can give e-
Potential to develop health products (still new)
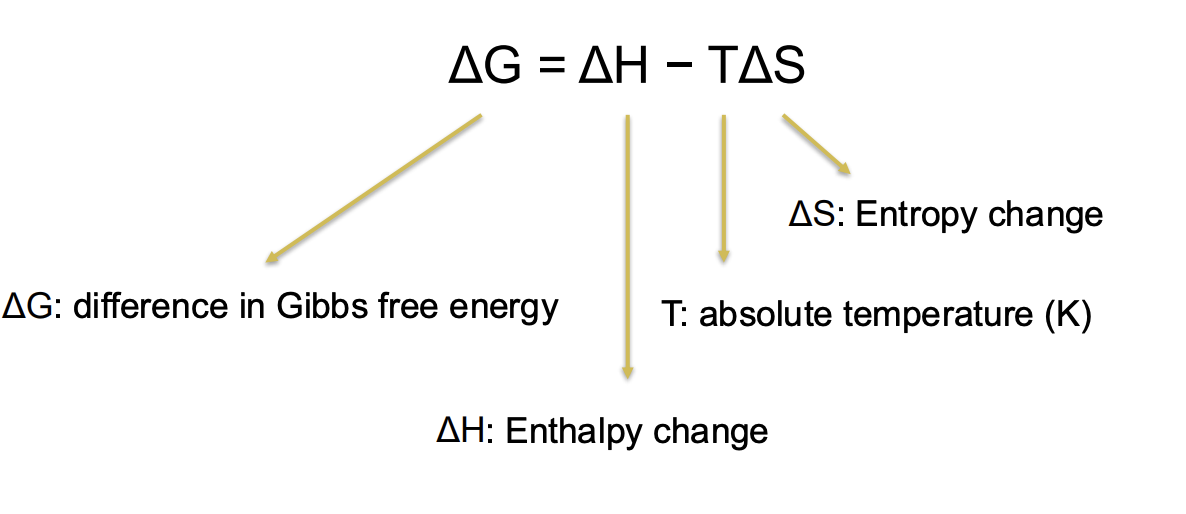
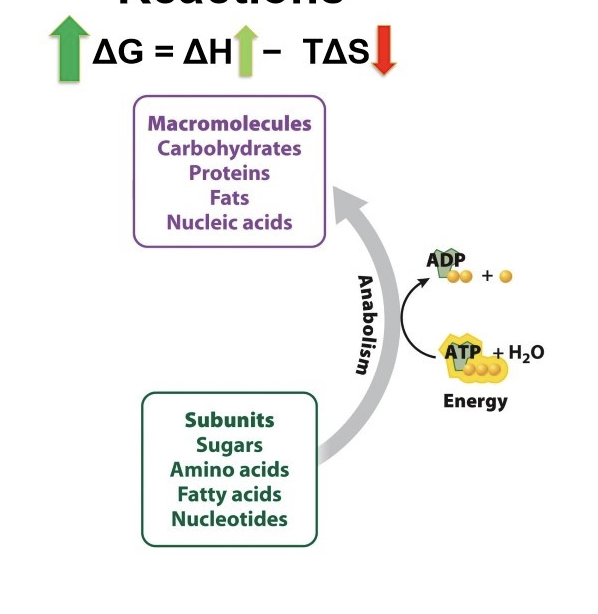
What is Gibbs free energy?
the amount of energy in a system available to do work
difference between products and reactents
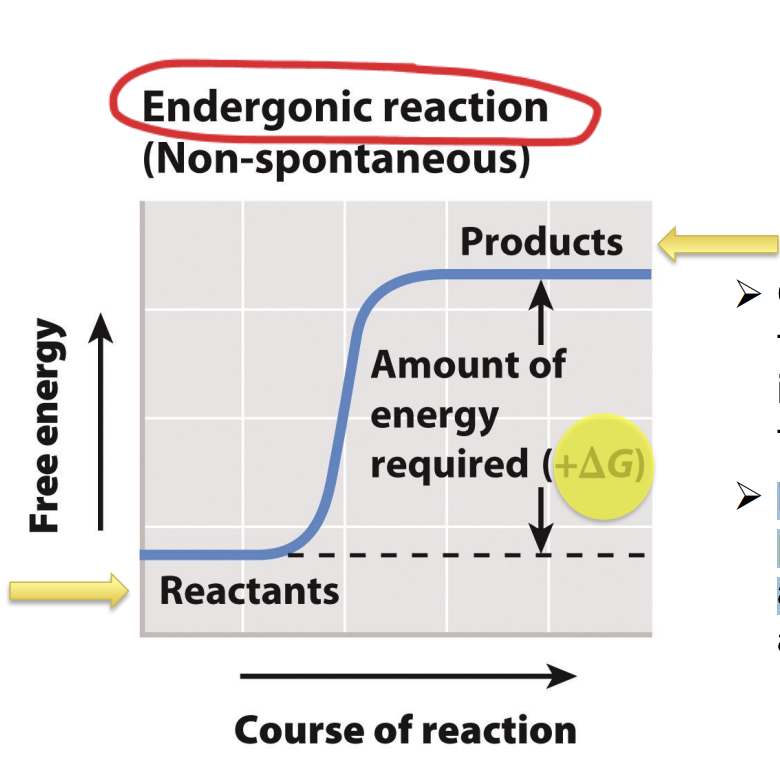
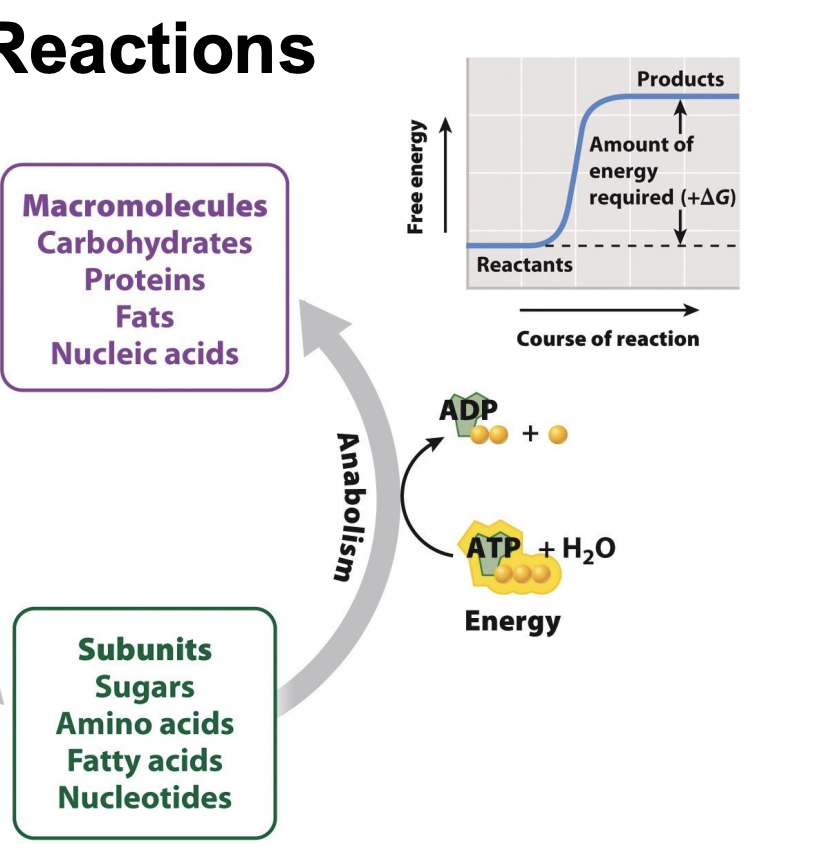
What are endergonic reactions?
Reactions with a positive ΔG require an input of energy
Products have more free energy than reactant: ΔG is positive
Positive: requires input of energy (endergonic reaction)
Endergonic (need ATP to start) -> non-spontaneous (need energy to activate it)
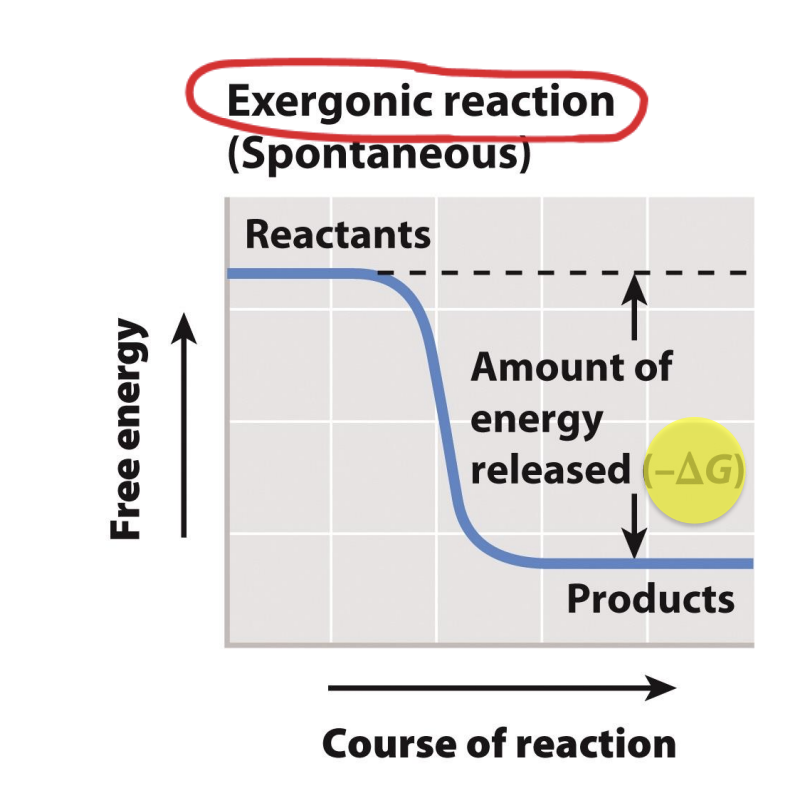
What are exergonic reactions?
Reactions with a negative ΔG release energy
Reactant has more free energy -> Negative
Negative: release energy (exergonic reaction)
Exergonic -> spontaneous (does not need extra energy to activate)
What is enthalpy (slide is confusing)?
The total amount of energy in a system.
interested in the energy available for a cell to do work, or G
Total energy or enthalpy (H) = energy available to do work (G) + energy lost to entropy (TS)
T- absolute temperature in Kelvin
S - entropy
G = H - TS
What is the equation of ΔG?
2nd law of thermodynamics
Energy lost due to randomness
Temperature is constant
compare the total energy and entropy of the reactants with the total energy and entropy of the products to see if there is energy available to do work.
useful way to see if a chemical reaction takes place spontaneously and whether net energy is required or released
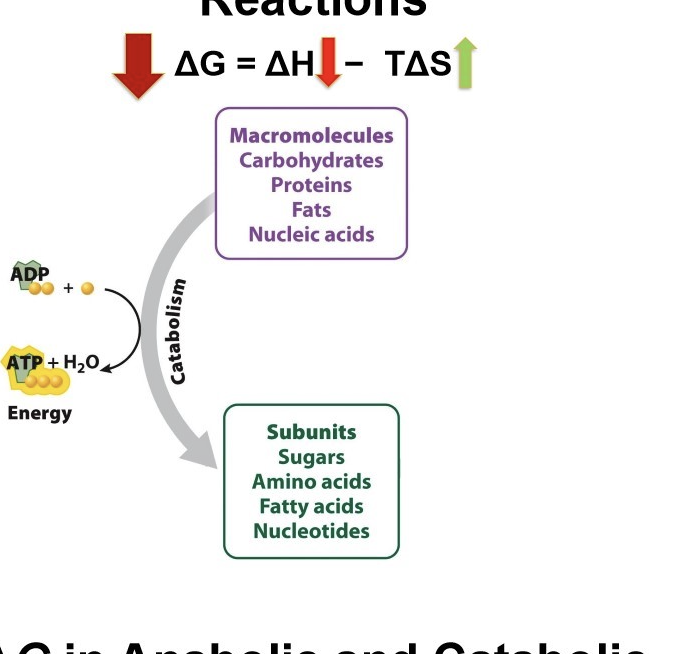
What is ΔG in catabolic reactions?
macromolecules(have higher energy level)
G is negative
reactants have more energy that products
decrease in enthalpy, products have more subunits → more disorder = higher entropy
Catabolic reaction is spontaneous and release energy (ATP)
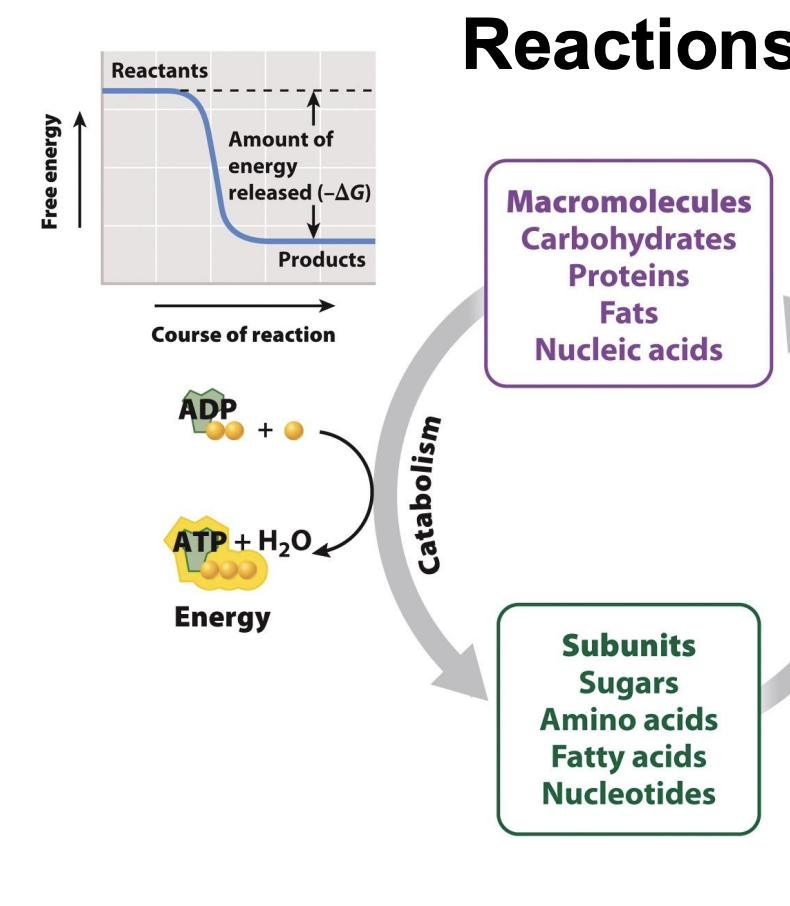
What is ΔG in anabolic reactions?
Positive G
Energy in products (bigger molecules) higher than reactants
Increase ΔH (total energy)
Products more organized -> decrease entropy
Requires energy input
non-spontaneous
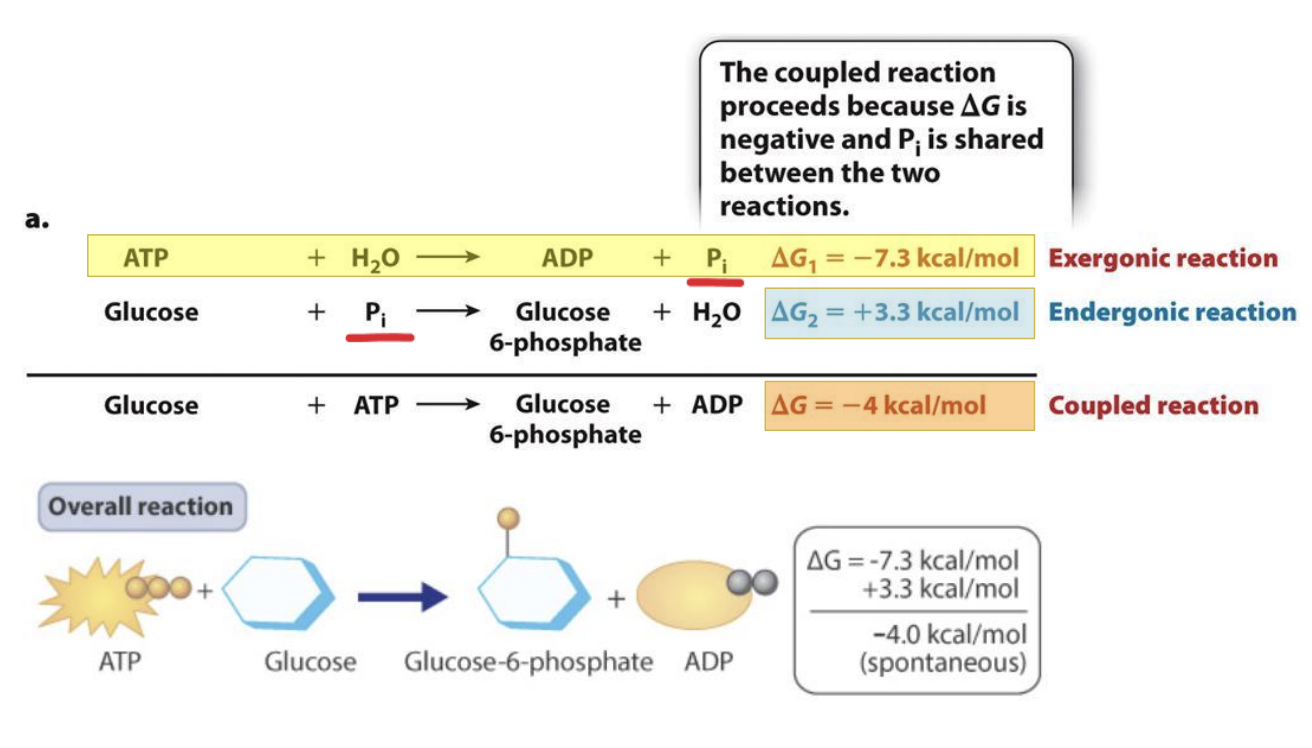
What is energetic coupling?
Reactions in the body can have multiple stops
is a process in which spontaneous reactions (ΔG < 0) drive a non-spontaneous reaction (ΔG > 0)
If sum of reactions have a negative G --> reaction will also be spontaneous
Reaction 2 has enough energy to activate reaction 1 -> coupled reaction can still occur
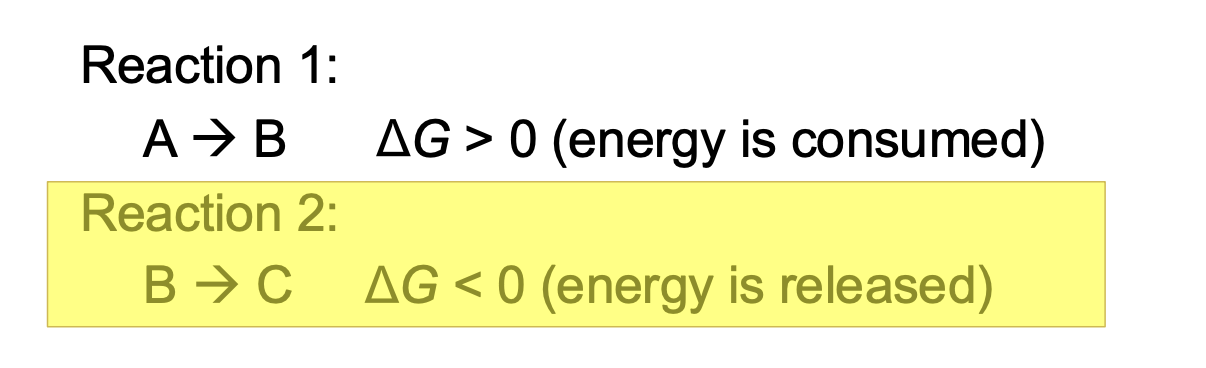
What do hydrolysis reactions often do?
break down polymers into their subunits → one product gains a proton and the other gains a hydroxyl group.
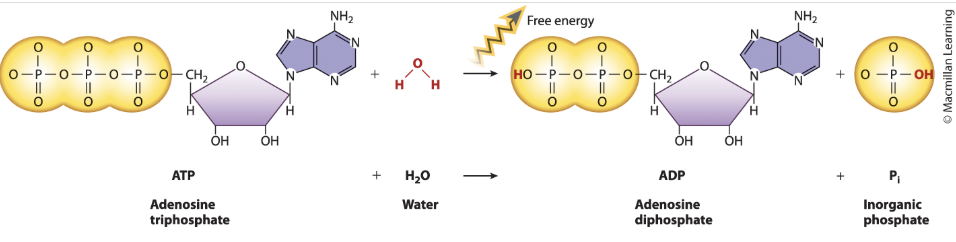
What is hydrolysis of ATP?
ATP + H20 → Pi(inorganic phosphate)(HPO4-2) + ADP
chemical reaction in which a water molecule is split into a proton (H + ) and a hydroxyl group (OH − ) .
Exergonic:
less free energy in the products than in the reactants.
Delta H
Phosphate groups of ATP negative → ATP has 3
ADP has 2 → more stable (contains less chemical energy in its bonds) than ATP → value of Delta H neg
Delta S: positive
Single molecule of ATP broken into two molecules (ADP and Pi)
Delta G
spontaneous and releases energy
How does energetic coupling occur in ATP hydrolysis?
ATP hydrolysis and formation of glucose 6 phosphate in cellular respiration
Inorganic phosphate shared between the reactions
Product of one has to be used as input to the next reaction for this to occur
Reaction formed by the coupling will still occur and be spontaneous
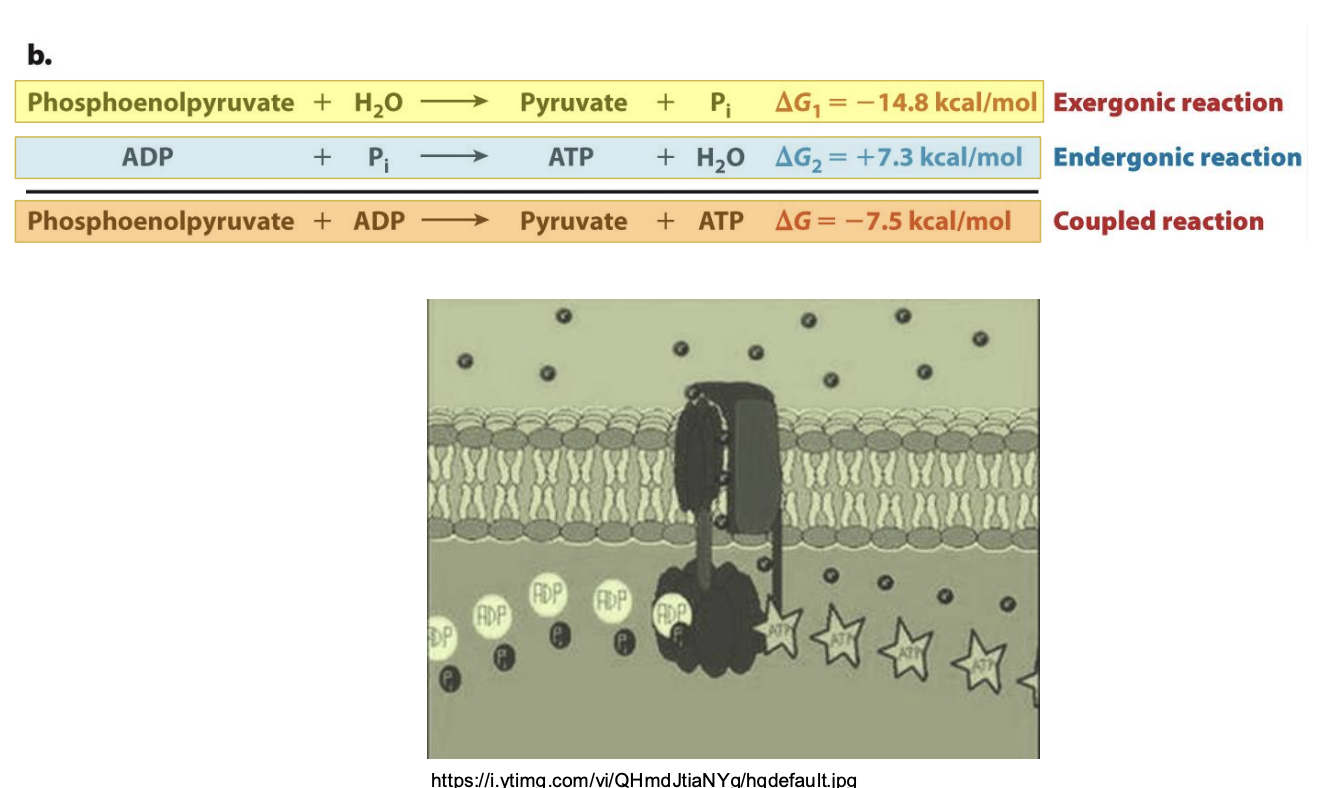
How does energetic coupling occur for hydrolysis of phosphoenolpyruvate?
Hydrolysis of phosphenoalpyruavte that drives synthesis of ATP
Cell needs to replenish ATP to carry out additional chemical reactions
Inorganic phosphate both input and output -> need phosphate group to synthesize ATP
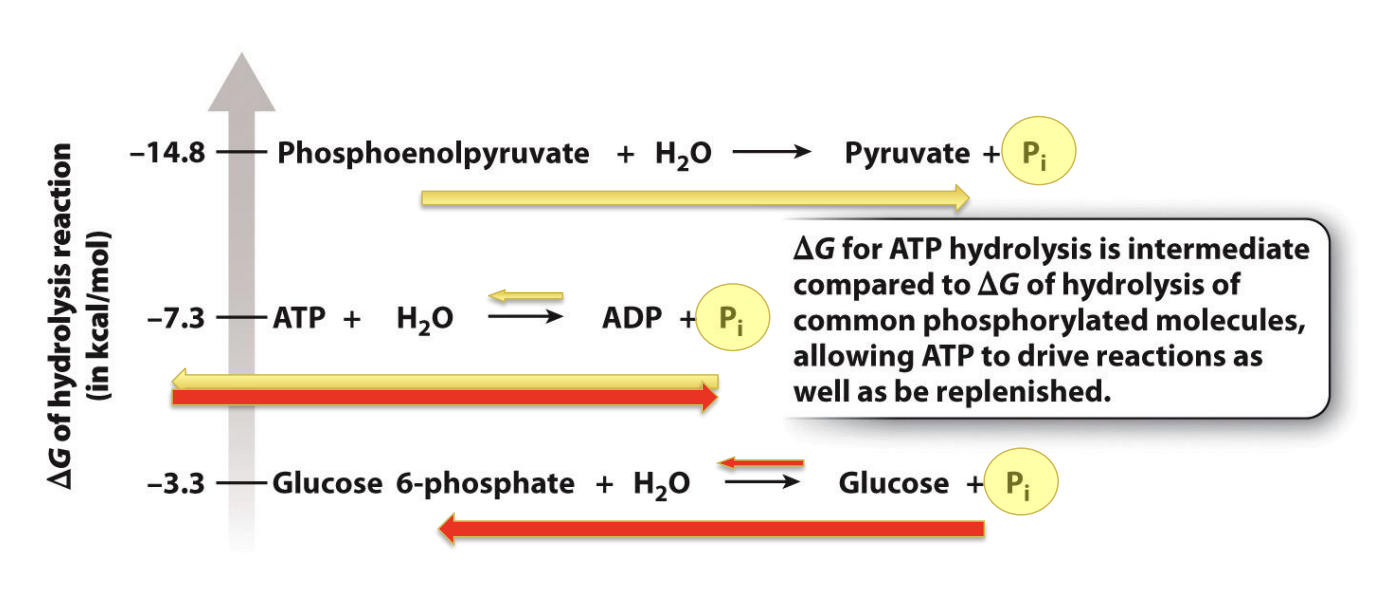
What is the ΔG of ATP considered?
Allows ATP to drive reaction when being abundant or can be managed when low (intermediate)
Reaction of hydrolysis of phosphoenolpyruvate have more negative G than ATP hydrolysis and first two reaction -> produce the ATP by donating a phosphate group to ADP -> will synthesize ATP
ATP hydrolysis has an intermediate energy difference
Flow of reaction is indicated by yellow arrow
When ATP is high -> drive other reaction with a less negative G than ATP hydrolysis
Glucose will reactive a phosphate group from ATP -> form glucose 6 phosphate
Flow: red arrows
ATP is consumed and creates ADP and inorganic phosphate group
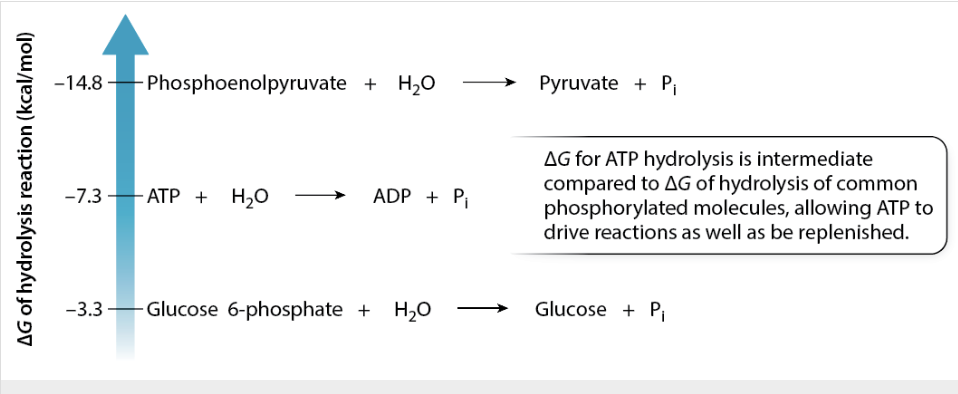
What is ATP and ADP cycling?
reactions that have a Δ 𝐺 more negative than that of ATP hydrolysis transfer a phosphate group to ADP by energetic coupling
reactions that have a less negative Δ 𝐺 receive a phosphate group from ATP by energetic coupling.
Therefore, ADP accepts a phosphate group (and energy), and ATP donates a phosphate group (and energy) as it cycles between ATP and ADP.
ATP–ADP cycle is at the core of energetic coupling between catabolic and anabolic reactions
Why is ATP useful?
Dynamic system that depends on wether there is enough ATP or not
Body can use different reactions to create or consume ATP
Hydrolysis reaction is intermediate
Other molecules are not intermediate so they cannot be used as currency of energy
intermediate ΔG
1st example: ATP is consumed
2nd: ATP is synthesized
ATP useful because it can consumed and synthesized
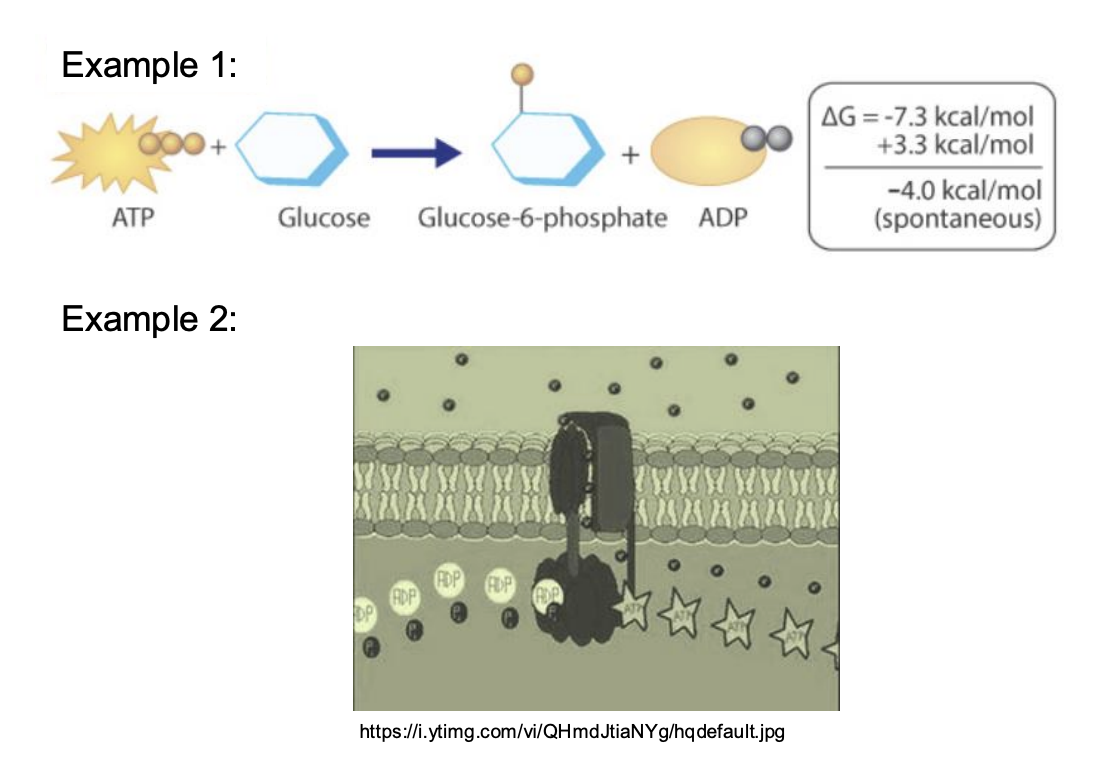
What does both exergonic and endergonic reactions both need?
all chemical reactions require an initial input of energy to proceed
exergonic: the energy released is more than the initial input of energy → net release of energy.
What is a transition state?
New compound forming -> brief transition state where old bonds are breaking and new bonds are forming
Transition state (unstable) -> has lots of free energy
highest free energy value = transition state
Example
Carbonic acid formation from CO2 and water
Both reactants are most stable than transition state
Reaction requires input of energy to reach the transition state (EA)
Need energy to put it two work
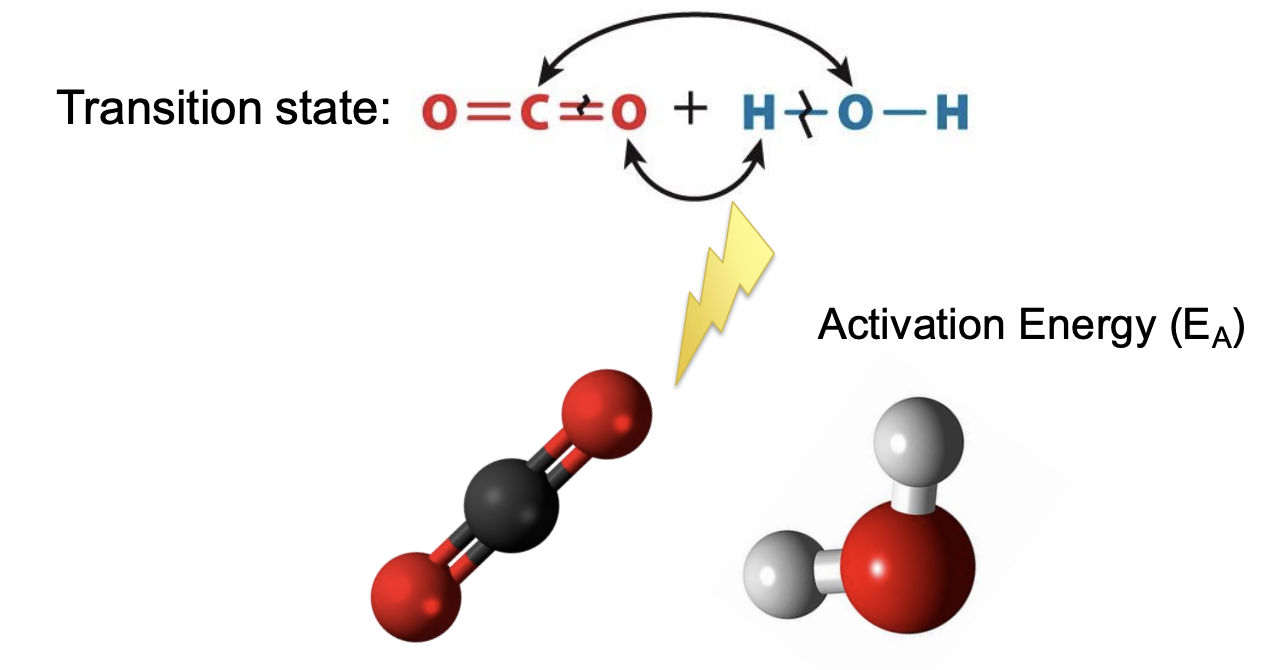
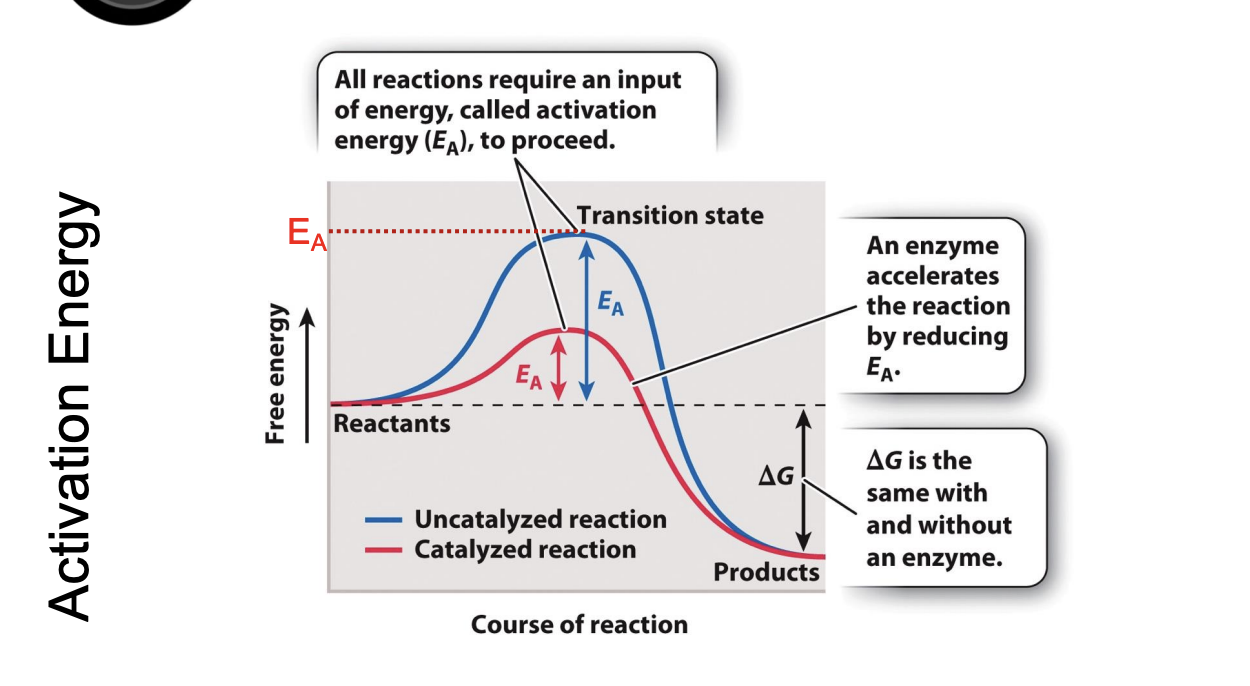
What is activation energy (EA)?
all reactions require an input of energy to proceed
energy input necessary to reach the transition state
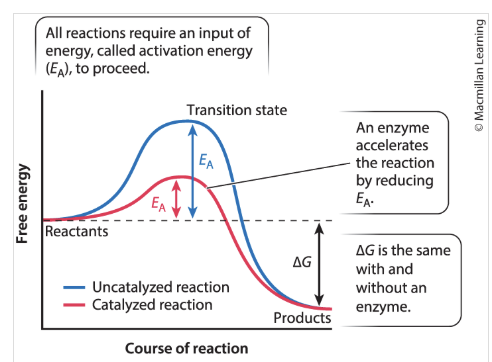
What happens after the transition state is reached (b)?
the reaction proceeds
products are formed,
energy is released into the surroundings (the downhill portion of the curve)
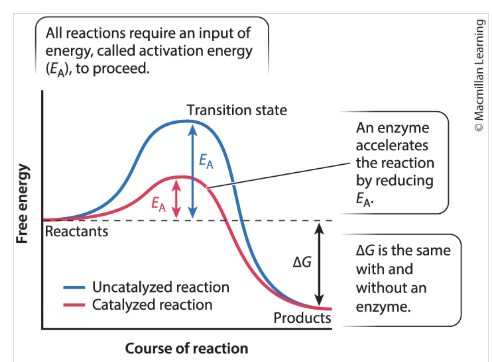
What are enzymes?
protein catalysts that can increase the rate of biochemical reactions by stabilizing the transition state and decreasing free energy (decreasing curve)
accelerates the reaction by reducing E
lowers obstacle
Delta G is the same with and without the enzyme
What is a substrate?
In a chemical reaction catalyzed by an enzyme, the reactant is often referred to as the substrate
How does an enzyme decrease EA (I)?
Low Ea -> lower energy required for reaction to work
Enzyme: product of proteins
Catalyzes reaction by lowering EA
Substrate (S) is converted to Product (P)
In presence of E → substrate forms couples with the enzyme (enzyme-substrate, ES)
ES converted to enzyme product (EP)
Last: complex disassociates and releases enzyme and product
Enzyme forms complex with substrate ES (enzyme-substrate complex)
Requires less EA to start reaction
Substrate is changed into different product (ES) -> structure also changes
Substrate is converted into product while still working with enzyme complex -> change to EP (enzyme product complex) -> complex dissociate and release enzyme (impacts shape of product)
Enzyme does not change, substrate of product changes
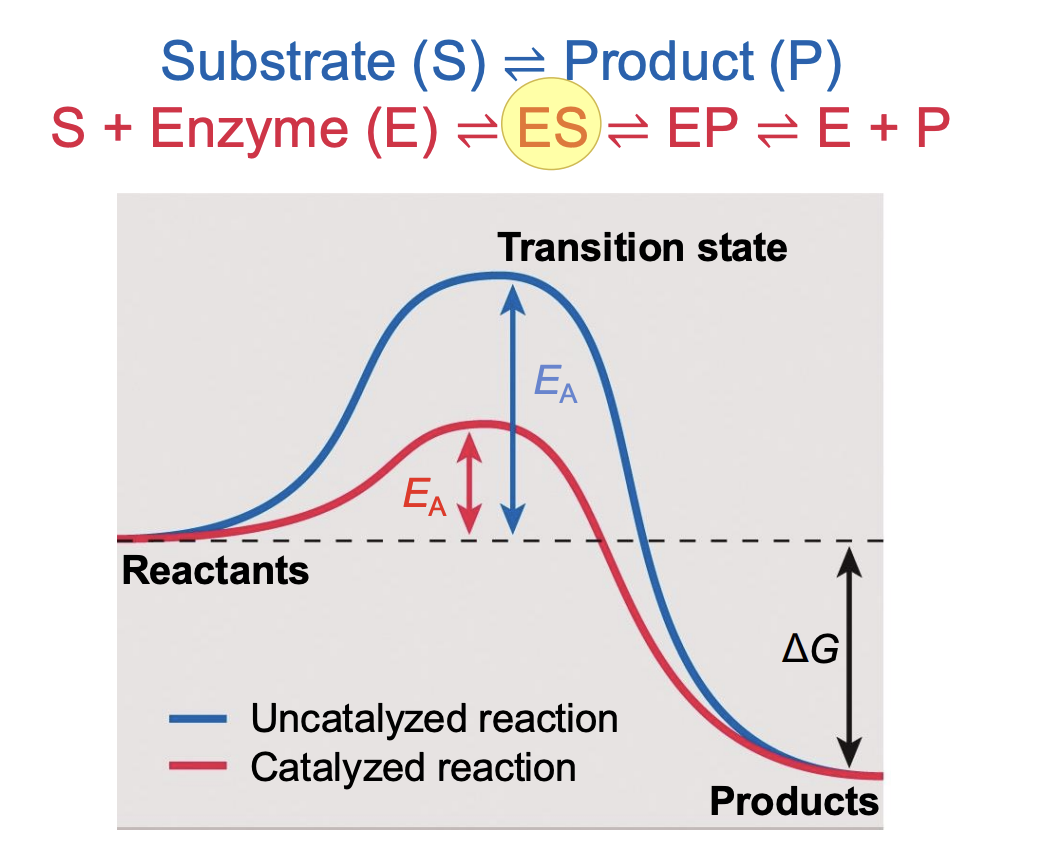
What do specific interactions between enzyme and the substrate in the complex do?
stabilize the transition state → reduce activaiton energy
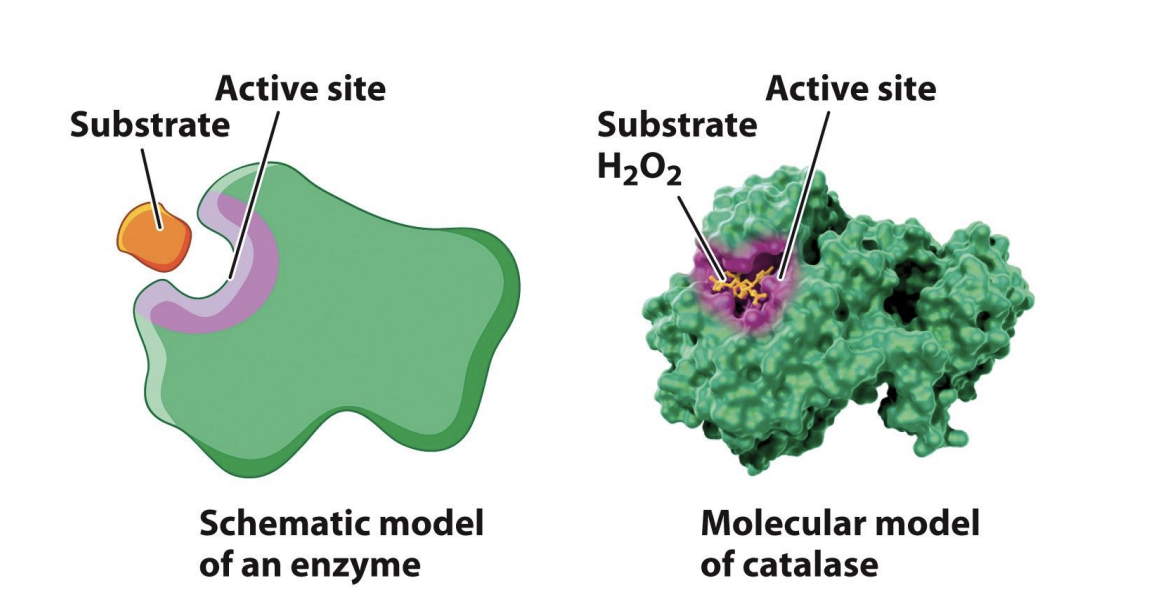
How are active sites formed?
Enzyme active site is small compared to enzyme itself
Only need small cavity to combine with substrate
active sits made of amino acids → only a few amino acids actually catalyze (b)
Active amino acids can be spaced far apart in peptide chain
Peptide are synthesized will have few sites that will work an active site when folded together
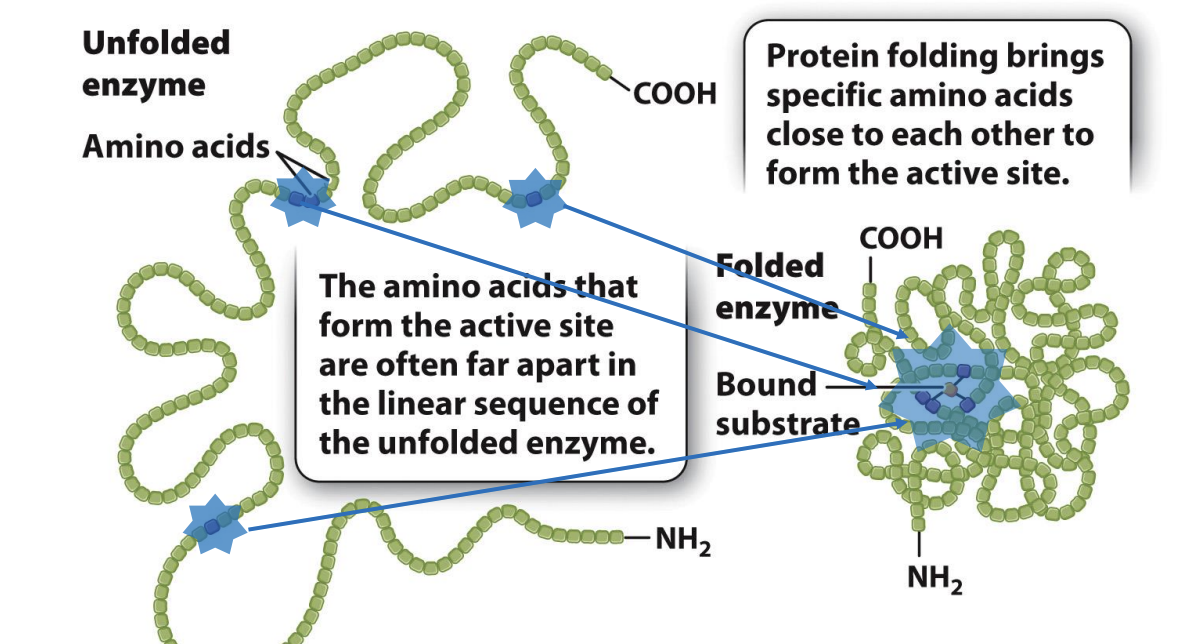
Why are enzymes so large?
because each of these amino acids occupies a specific spatial position to align with the reactive group on the substrate.
If the few essential amino acids were part of a short peptide, the alignment of chemical groups between the peptide and the substrate would be difficult
the length of the bonds and the bond angles in the peptide would constrain its three-dimensional structure.
How is enzyme shape changed?
The enzyme active site binds the substrate and converts it to the product.
The interactions between the substrate and the active site will decrease the activation energy required for the reaction
Enzymes are proteins with active sites to facilities reactions
ES complex will meet active site to combine with reactant
Active site will change reactent to products
Interactions between substrte and active site -> decreases EA
Enzymes needed by many biological reactions -> not enough EA to make them happen → they go slower
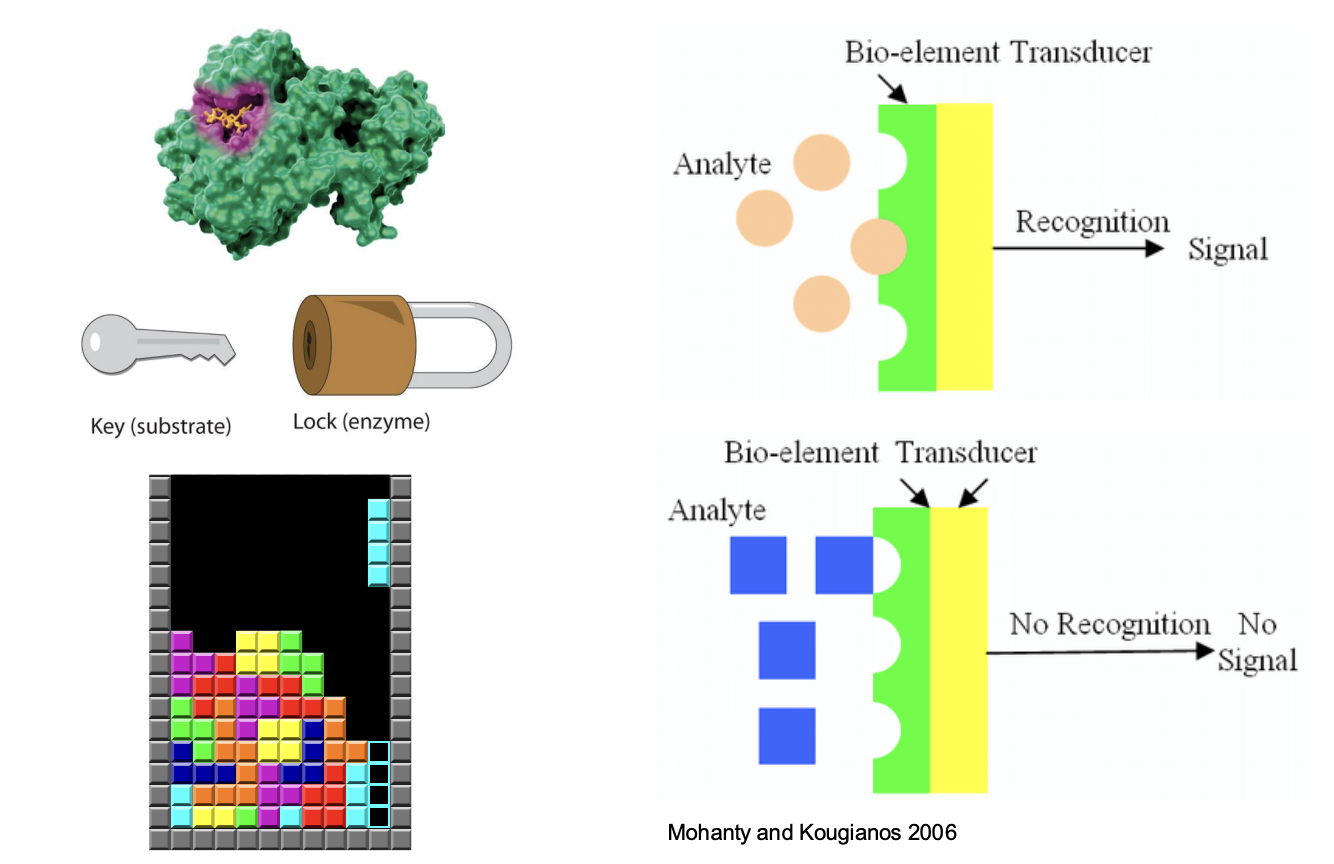
How does enzyme specificity work?
Enzyme specific for certain substrates (structure of active sites)
induced fit mode: shape of substrate may influenced by binding to active site
fit becomes closer as substrate and enzyme interact → not rigid structures → mold to some degree to each other
Specificity of enzyme is the basis of biosensors / bioengineering
Signals released when only the exact biological molecules can be detected
Once they have identified and combined with certain molecule -> release signal
Wrong binding -> cannot bind -> no following reaction -> no signal
What is an activator?
A compound that increases the activity of an enzyme;
also, a protein that binds to a DNA sequence and turns on transcription.
What is an inhibitor?
A compound that decreases the activity of an enzyme.
What are the types of inhibitors?
Reversible or irreversible depening on how they interact with enzyme
Covalent or ionic bonds -> strong -> inhibition is irreversible and cause dmg
Weak intermolecular -> reversable
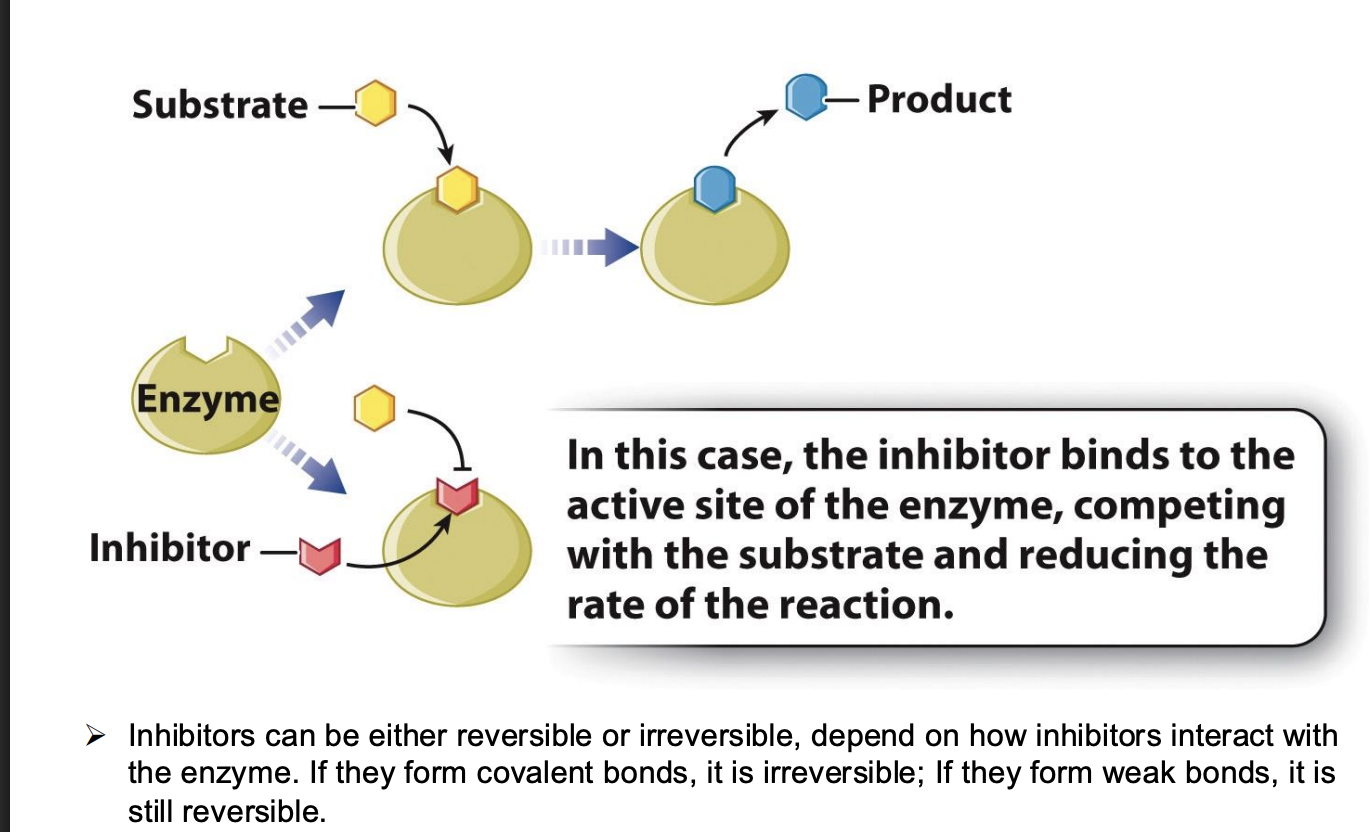
What is a competitive inhibitor?
binds to active site -> substate can not form enzyme substrate complex anymore -> reaction is inhibited
Share same active site
Usually similar structure as substrate to compete with it for active sites
What is an allosteric site?
a binding site on an enzyme or receptor that is distinct from the enzyme's active site
What is a non-competitive inhibitor?
active site + allosteric site
inhibitor binds with alternative site
Usually have diff structure than substrate
Binding of inhibitor to enzyme will change shape of enzyme
Active site also changes
Substrate not able to combine with active site
Can be influenced by inhibitor
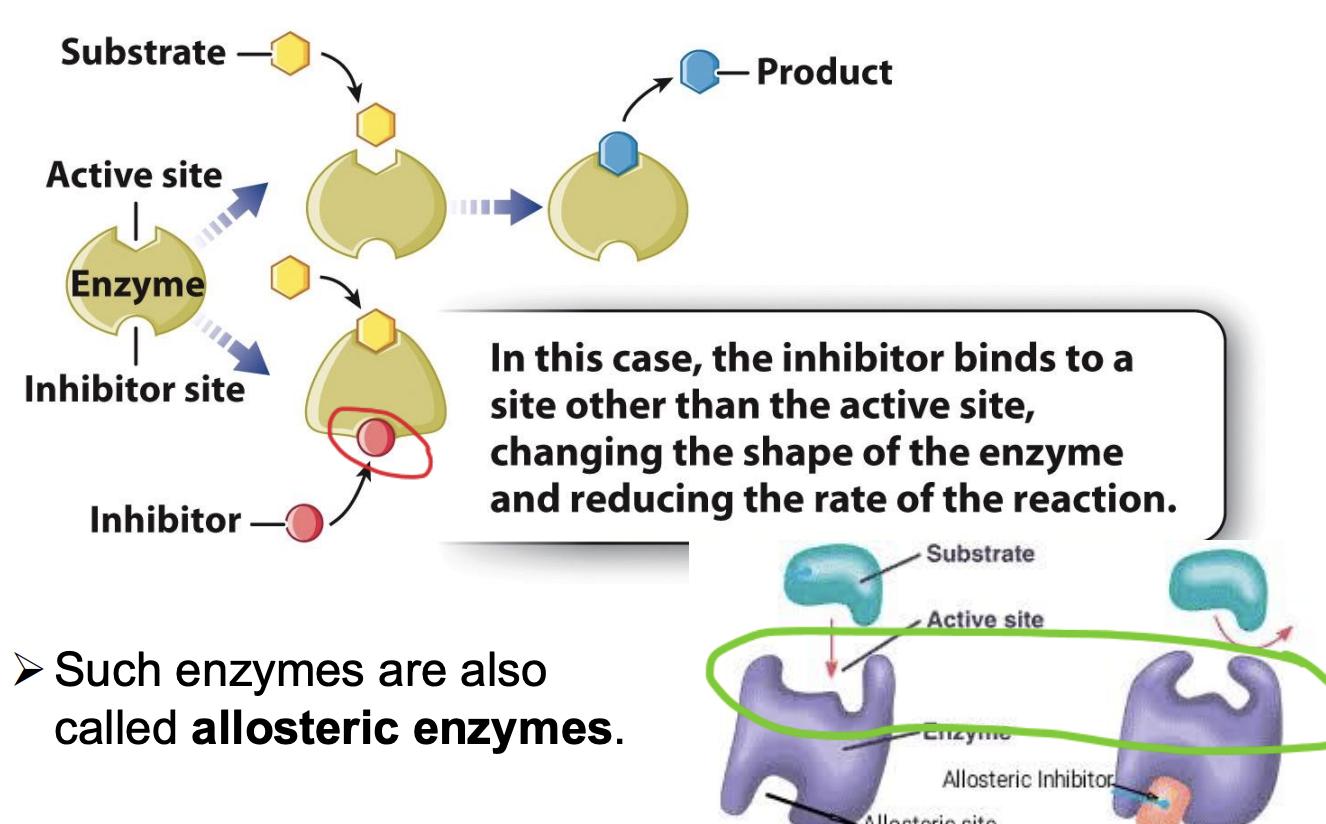
What are allosteric enzymes?
an enzyme that is activated or inhibited when binding to another molecule changes its shape.
Enzymes that are regulated by molecules that bind at sites other than their active sites
What can the activity of allosteric sites be influenced by?
inhibitors a
activators
What role do allosteric enzymes play?
Allosteric enzymes play imp role in chemical regulations of reaction (and metabolic pathway)
Reduce or stop amount of chemical reactions
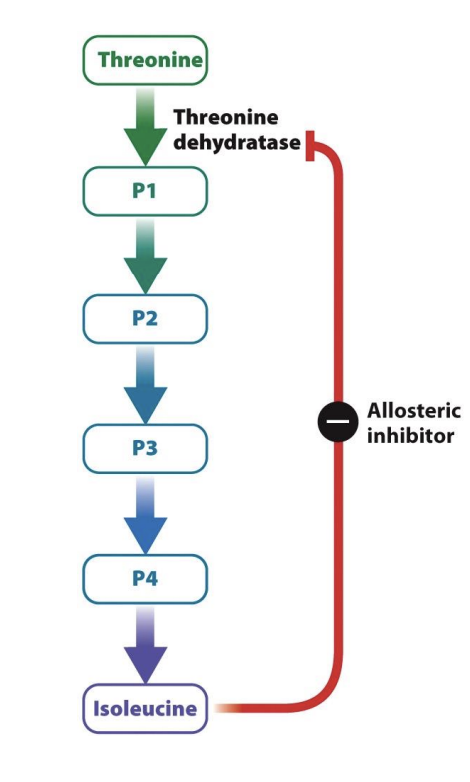
Allosteric enzyme example
bacterium has enough isoleucine → shut down pathway to not waste energy by synthesizing more → cell relies on enzyme inhibitor
synthesize of isoluencine (inhibitor), reactant is threonine
5 reactions
P1-P4: intermediate products
Once enough isoleucine (product) is made -> whole process will be inhibited by product itself
Isolucine is allosteric inhibitor
Extra isolunece will bind to first enzyme in path way (theorine dehydrates) at a site distinct from the active site
theorine = allosteric enzyme
binding of isoleucine -> changes shape of enzyme and inhibit further production from the beginning
Negative feedback: uses products as allosteric inhibitor to stop reaction from beginner
final product inhibits the first step of the reaction
What does the rate of reaction depend on (b)?
activity of threonine dehydratase = rate of the reaction → depends on the concentration of substrate
low concentration of threnonine = low reaction rate
high concentration = enzyme activity increases
At a particular threshold, a small increase in threonine concentration results in a large increase in reaction rate.
Finally, when there is excess substrate, the reaction rate slows down.
Why are some mushrooms toxic to human beings?
Amatoxins are selective inhibitors of RNA polymerase II, which is a vital enzyme in the synthesis of messenger RNA (mRNA), microRNA, and small nuclear RNA (snRNA).
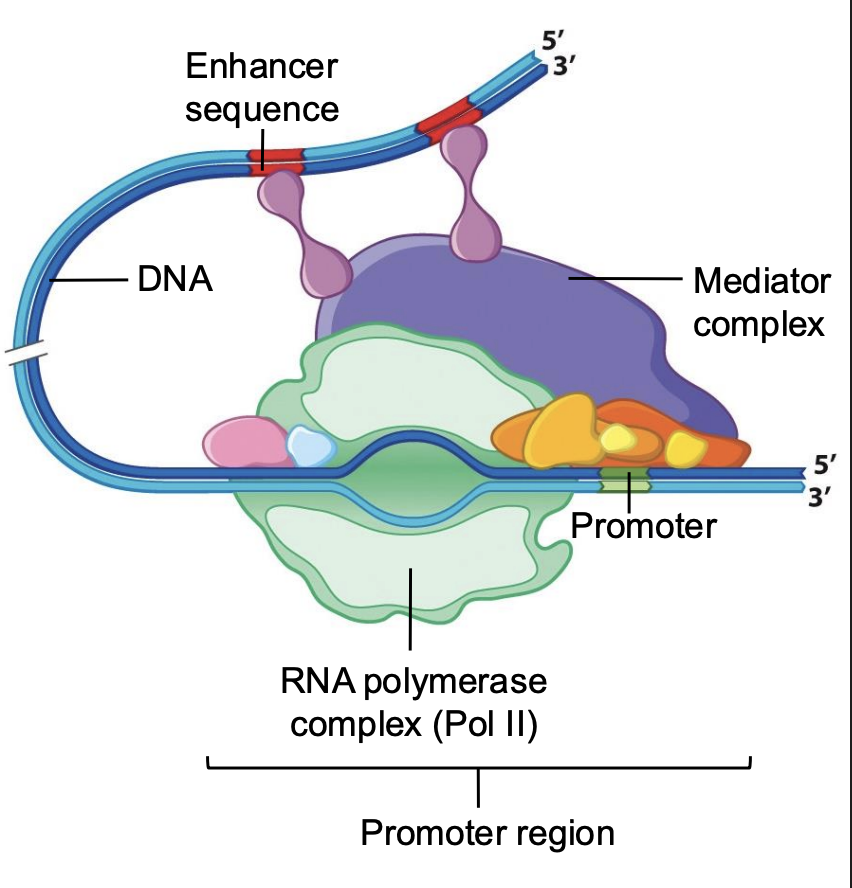
Why is RNA polymerase complex (Pol II) important?
RNP II important for transcription and protein production
Cover image
How RNP works in body to make RNA
RNP II needs to bind directly to DNA template to make RNA
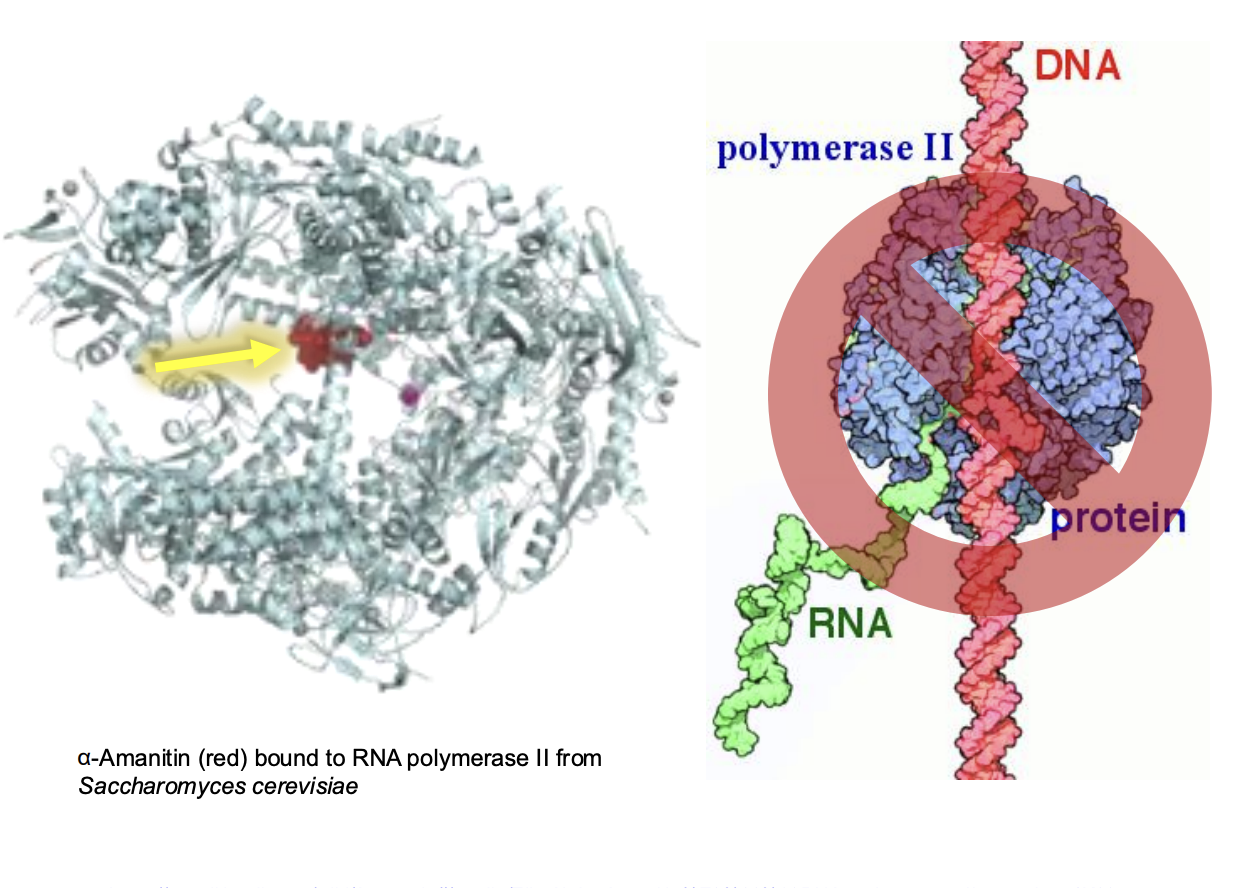
How does amatoxins work to be inhibitors?
Amatoxin (red) combine to RNP II -> enzyme cannot bind to DNA template (competitive inhibitor)
Toxin can travel through stream and reach other organs -> dmg to liver, heart, brain
Inhibition + organ failture = irreversible
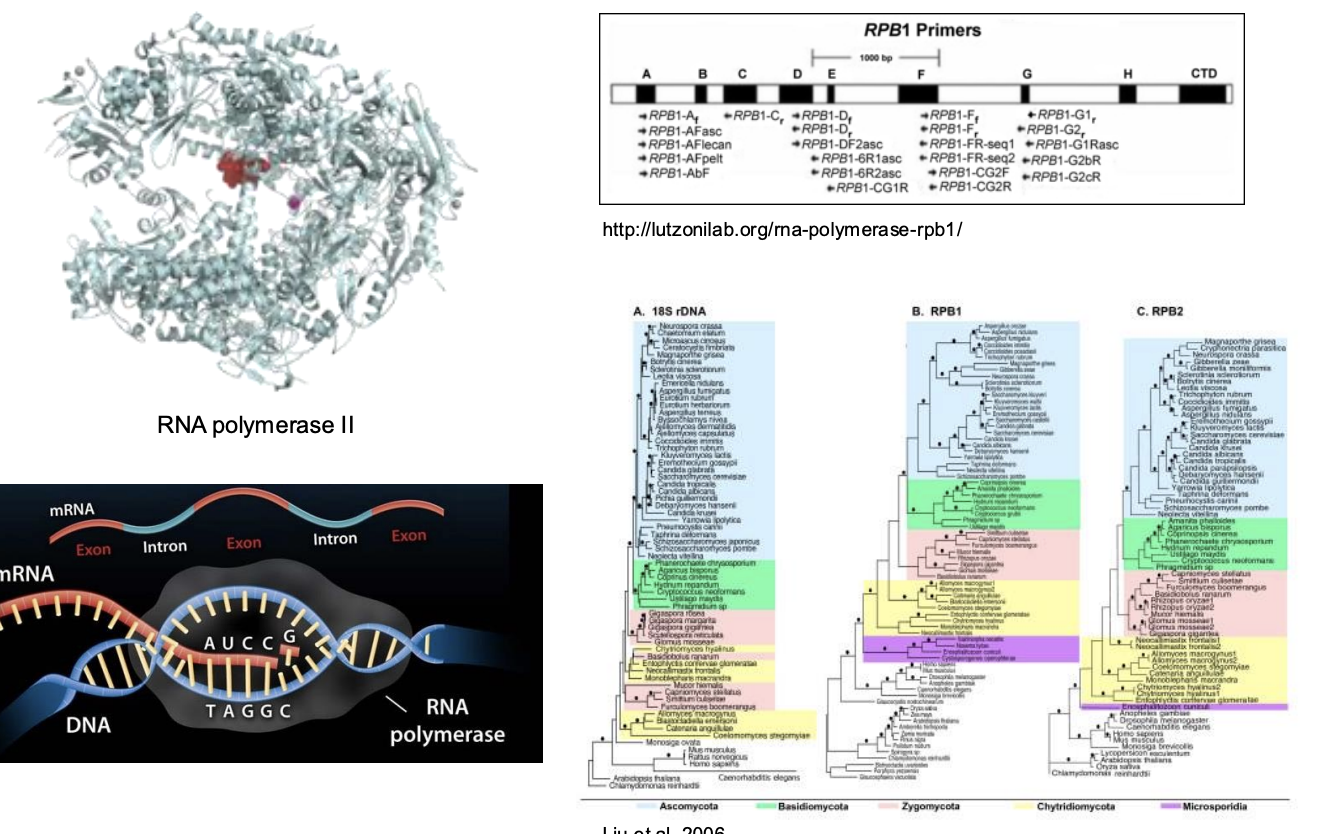
What is the phylogenetic analyses of RNP II?
Many polyphyletic becomes monophyletic
RPP I and II have been used more and is more robust -> more popular
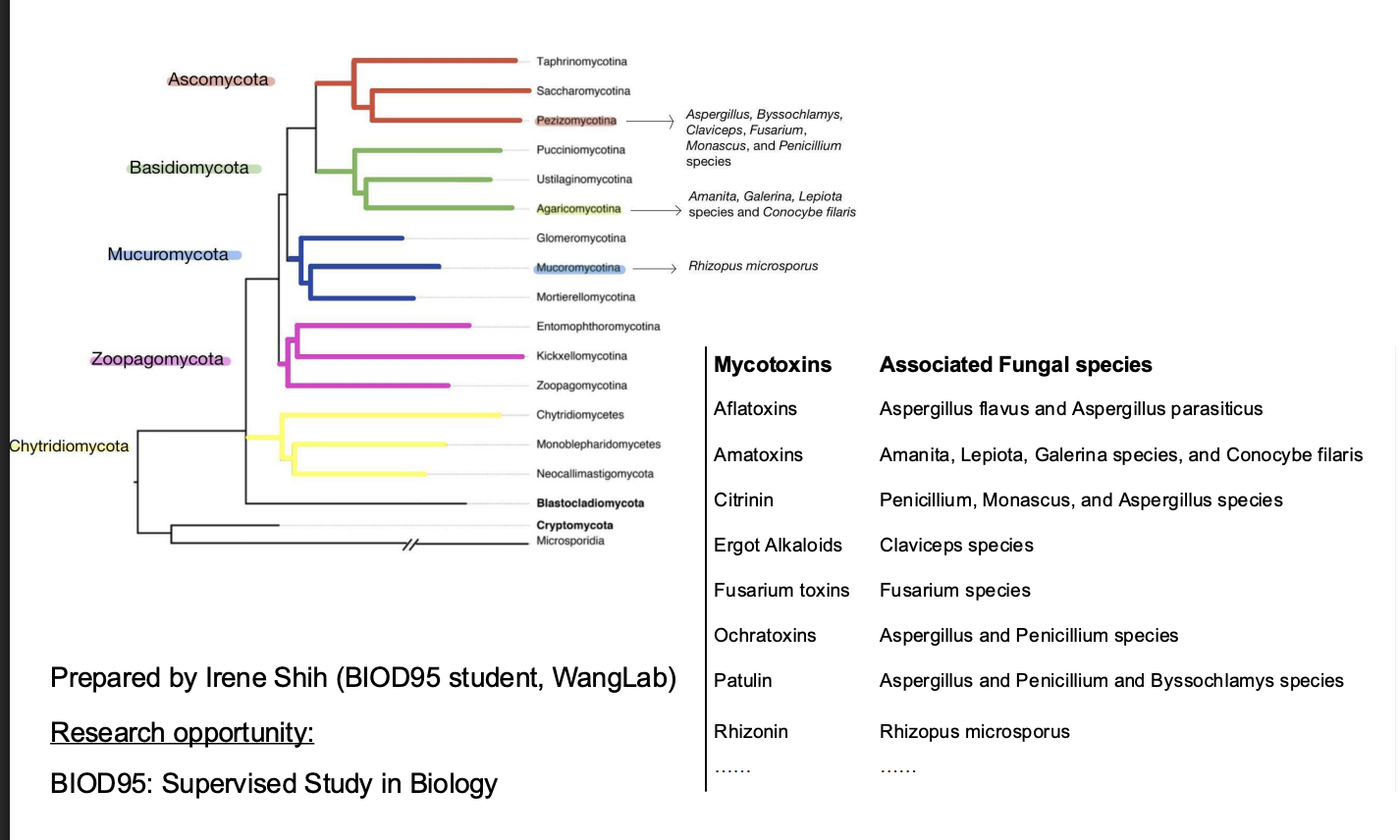
What are mycotoxins?
a toxic secondary metabolite produced by fungi
Many fungi can product toxin
Fungal tree of life
Diversity produced from a handful of fungal species