arenes physical properies and reactivity + electrophilic substitution
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
why do arenes have high melting points
benzene has a flat hexagonal molecules that pack closely together making it harder to seperate
what do arenes mix with
other hydrocarbons and non polar solvents
two factors that are important in reactivity of aromatic compounds
ring of high electron density, that has delocalised bonding and can be attacked by electrophiles
aromatic ring is very stable, delocalisation energy is needed to break ring
what feature does an aromatic compound produce
smoky flame
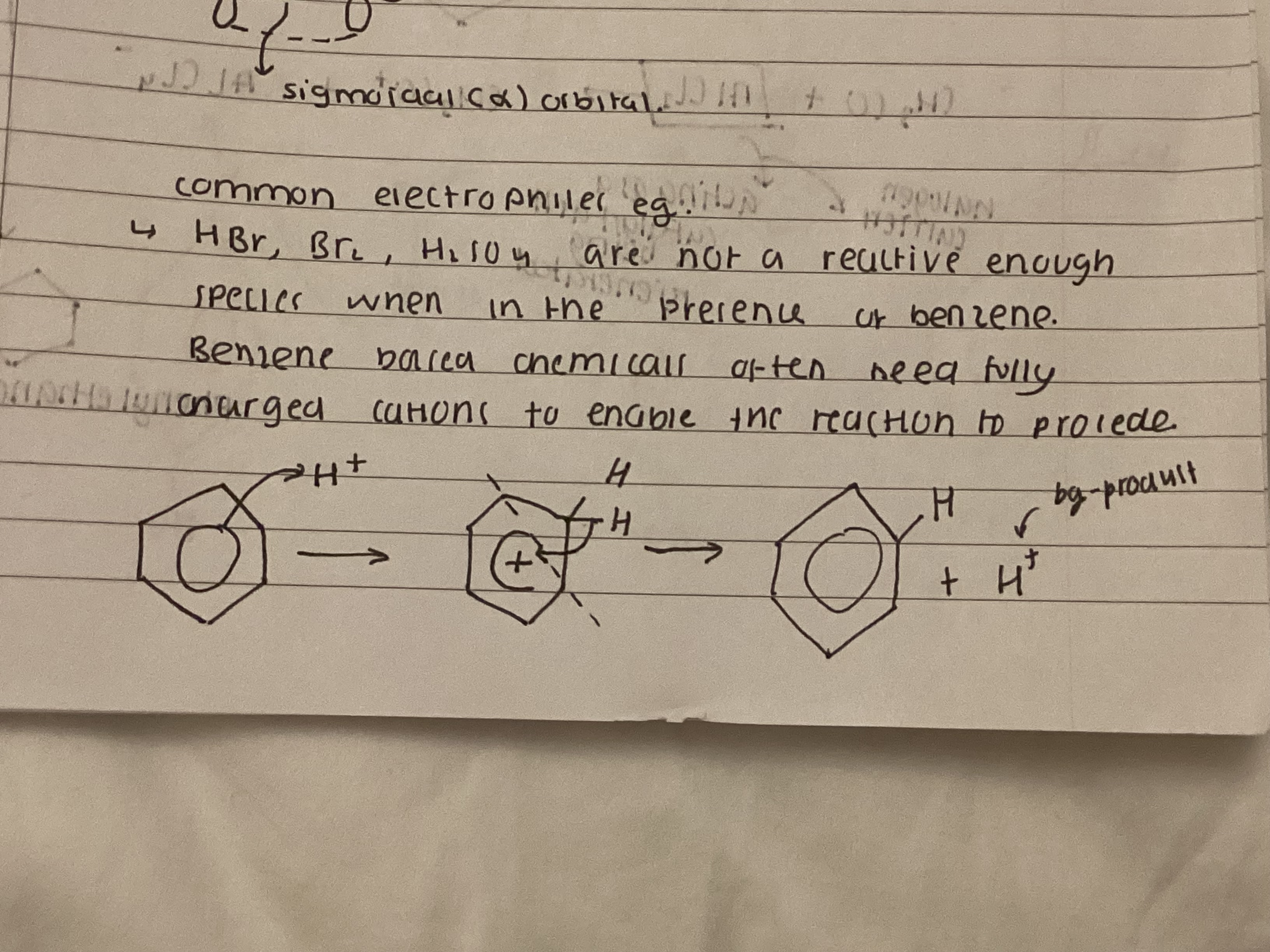
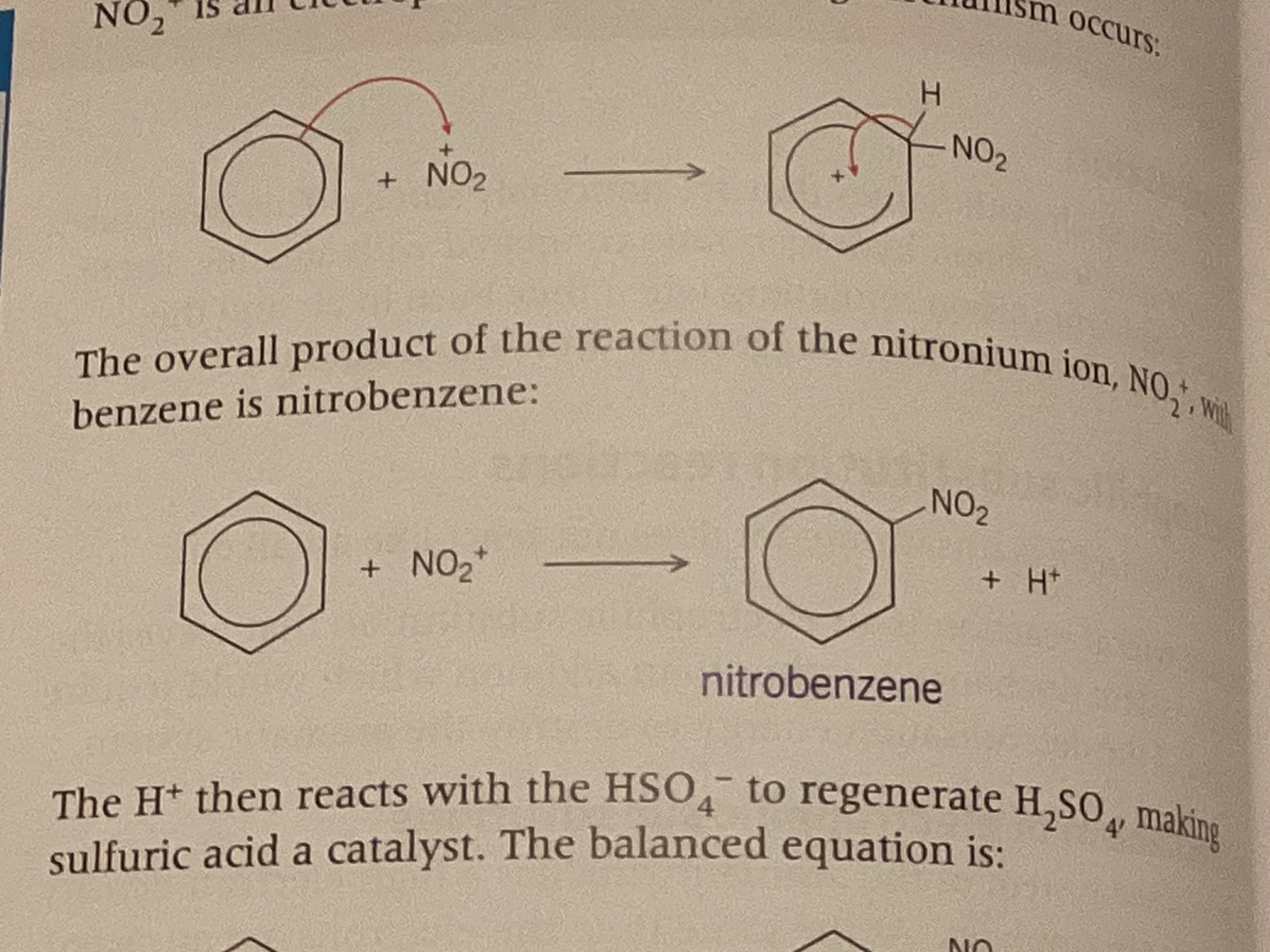
show electrophilic substitution
Nitration equation
H2SO4 + HNO3 → NO2+ + HSO4- + H2O
show nitration of benzene + name of product formed
nitrobenzene
why is sulphuric acid a catalyst
HSO4- reacts with H+ to regenerate H2SO4
importance of nitrated arenes
explosives + industrial dyes
What catalyst does the Friedel-Craft acylation reaction use
aluminium chloride cataylst
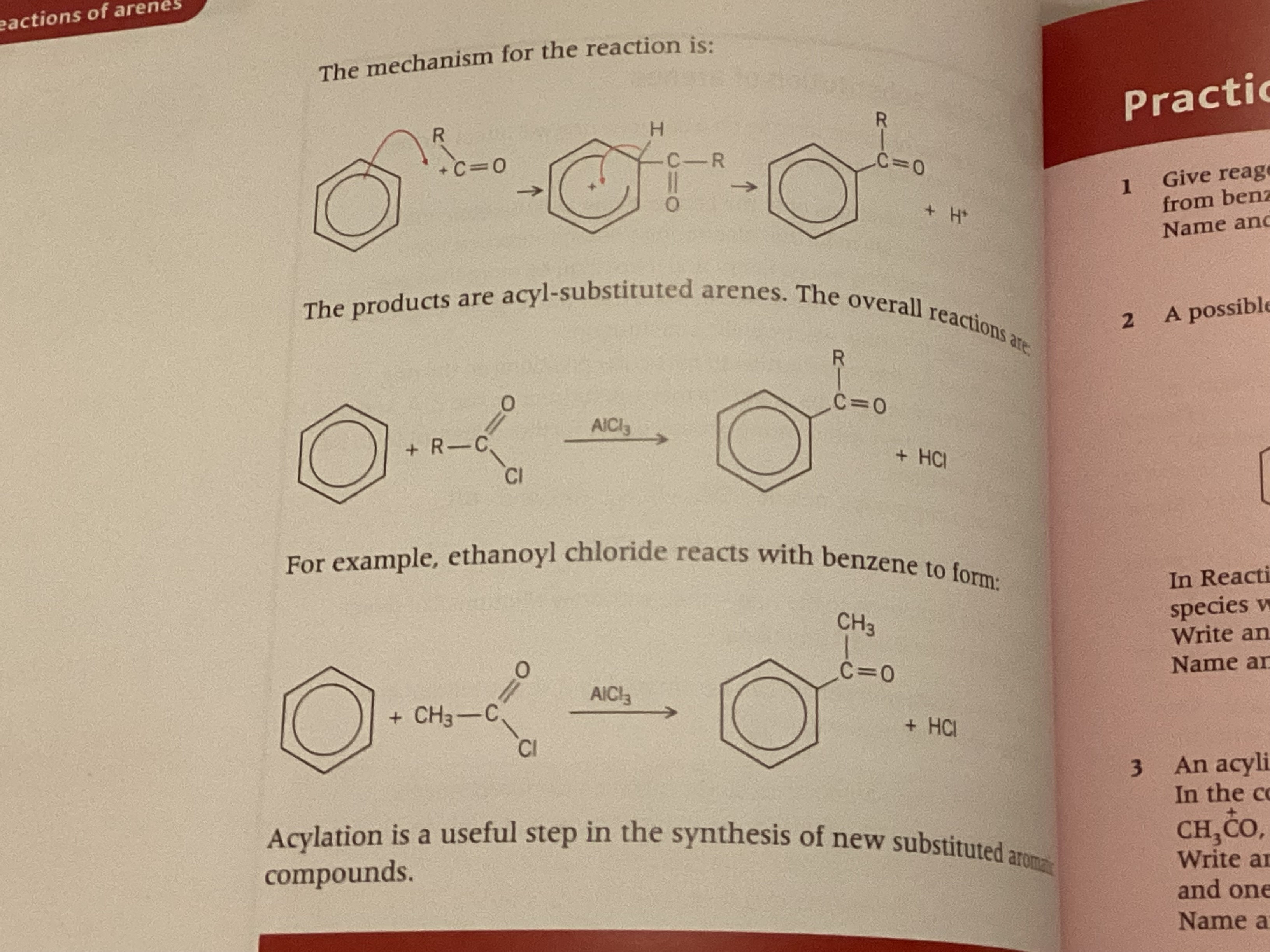
Show equation for friedel-craft acylation reaction
AlCl3 + RCOCl → RCO+ + AlCl4-
show friedel crafts reaction mechanism + name of product formed
phenylethanone