Chemical Bonding
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Variation method
Quantum Mechanics hydrogen atom ka exact solution de sakta hai, lekin helium (2 electrons) ya usse zyada electrons wale systems ke liye exact solution nahi nikal sakta. Problem hoti hai electrons ke beech repulsion (1/r₁₂ term) ki wajah se.
· Isliye, hum approximation methods ka use karte hain. Is chapter mein hum Variation Method padh rahe hain.
Variation Method ka Basic Idea:
· Maan lo kisi system ka correct wavefunction ψ₀ hai, toh uska exact energy E₀ hoga.
· Variation Principle kehta hai: Agar tum koi bhi dusra (trial) wavefunction ψ_i choose karte ho, toh usse calculate hone wala energy E_i hamesha correct energy E₀ se zyada ya equal hoga.
· E_i ≥ E₀
· Fayda: Hum ek aisa trial wavefunction lete hain jisme kuch parameters (jaise C₁, C₂) hote hain. Phir hum energy ko minimize karte hain in parameters ke respect mein. Aisa karne se hum actual energy aur wavefunction ke bahut close pahunch jaate hain.
· Success isi par nirbhar karti hai ki aap kitna accha trial function choose karte hain.
5. Practical Meaning
Hum ek trial wavefunction choose karte hain (approx guess).
Usme kuch parameters (α, β, etc.) daalte hain.
Phir hum ko minimize karte hain un parameters ke respect mein.
Jis set of parameters pe energy minimum aata hai —🟢 wahi humara best approximate wavefunction aur energy hota ha

H2+ ion LCAO-MOT
H₂⁺ Ion (LCAO-MO Theory se)
· H₂⁺ ion mein hote hain: 2 protons aur 1 electron.
· Iska exact solution bhi nahi nikal sakte, isliye Variation Method ka use karte hain.
· Hum ek Molecular Orbital (MO) banate hain jo do Hydrogen atoms ke 1s Atomic Orbitals (AOs) ka Linear Combination hai (isliye LCAO-MO).
ψ = C₁.1sₐ + C₂.1s_b
Energy Calculate karna:
· Variation method use karke energy E ko C₁ aur C₂ ke terms mein likha jaata hai.
· Phir, lowest energy pane ke liye, E ko C₁ aur C₂ ke respect mein differentiate karte hain aur derivative ko zero set karte hain. Isse milti hain secular equations.
· In equations ko solve karne ke liye, hum ek secular determinant zero ke equal set karte hain.
Secular determinant solve karne par do energies milti hain:
1. Bonding Energy (E₁): E₁ = (Hₐₐ + Hₐb) / (1 + Sₐb)
2. Antibonding Energy (E₂): E₂ = (Hₐₐ - Hₐb) / (1 - Sₐb)
Yahan:
· Hₐₐ aur H_bb hain Coulomb Integrals (energy jab electron ek hi nucleus ke paas hai).
· Hₐb hai Resonance Integral (ye bond formation ko favourable banata hai).
· Sₐb hai Overlap Integral (ye batata hai ki do orbitals kitna overlap kar rahe hain).
Aur corresponding wavefunctions milte hain:
· Bonding Orbital (ψ₁): ψ₁ = (1sₐ + 1s_b) / √[2(1+Sₐb)] ...Symmetric, electrons nuclei ke beech concentrate hote hain.
· Antibonding Orbital (ψ₂): ψ₂ = (1sₐ - 1s_b) / √[2(1-Sₐb)] ...Antisymmetric, nuclei ke beech electron density kam hoti hai.
Doubt: ψ aur ψ same hai kya?
1. ψ aur ψ same hai kya?*
Short Answer: Haan, is case mein ψ aur ψ same hi hain.*
Detailed Reason:
· ψ ek wavefunction hai jo atomic orbitals (1sₐ, 1s_b) se bana hai.
· Hydrogen atom ke 1s orbitals real functions hote hain (complex nahi).
· Jab koi function real hota hai, toh uska complex conjugate (ψ*) function khud ke barabar hota hai.
· Isliye: ψ* = ψ
Isliye E ka formula mein ψ* ko ψ hi maana ja sakta hai.
Exception: Agar wavefunction complex parts rakhta (jaise p-orbitals ya magnetic fields mein), tab ψ* aur ψ different hote. But H₂⁺ ke simple case mein same hain.
Doubt:E ka formula aya kese

E ka formula derive kese

Secular equation ka derivation

Normalisation consideration for eqn (ii)
Ψ=C_1 1S_a + C_2 1S_b
Normalization Kya Hoti Hai?
Quantum mechanics mein, kisi bhi wavefunction ke liye probability overall space mein integrate karo toh 1 (100%) aana chahiye.
Kyun Important Hai Normalization?
· Probability: Ensure karta hai ki electron ko dhoondne ki total probability 100% ho
· Comparisons: Different wavefunctions ke energy values ko compare kar sakte hain
· Physical Meaning: Wavefunction ka square probability density deta hai, jo physically meaningful hona chahiye
Simple bhasha mein: Normalization yeh ensure karta hai ki humara wavefunction physically correct hai aur probability 100% hai ki electron kahi na kahi milega!
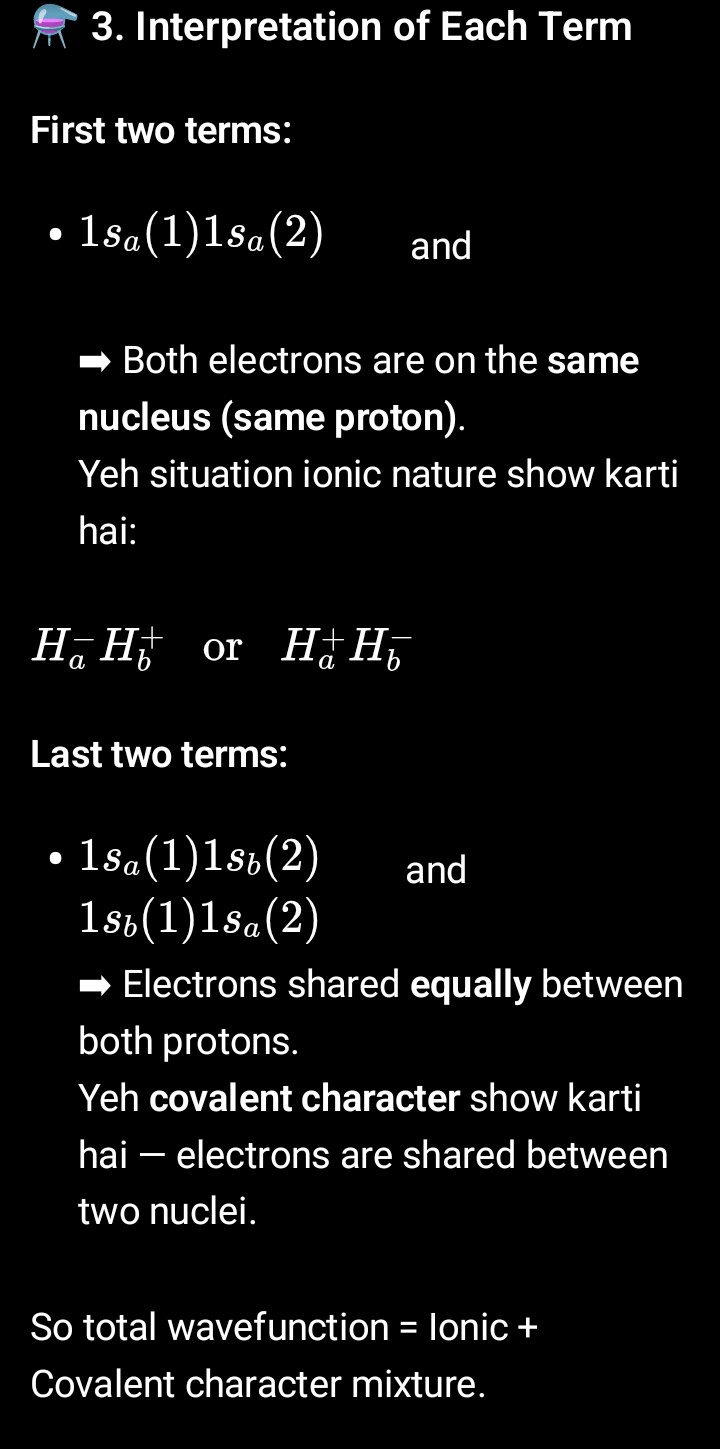
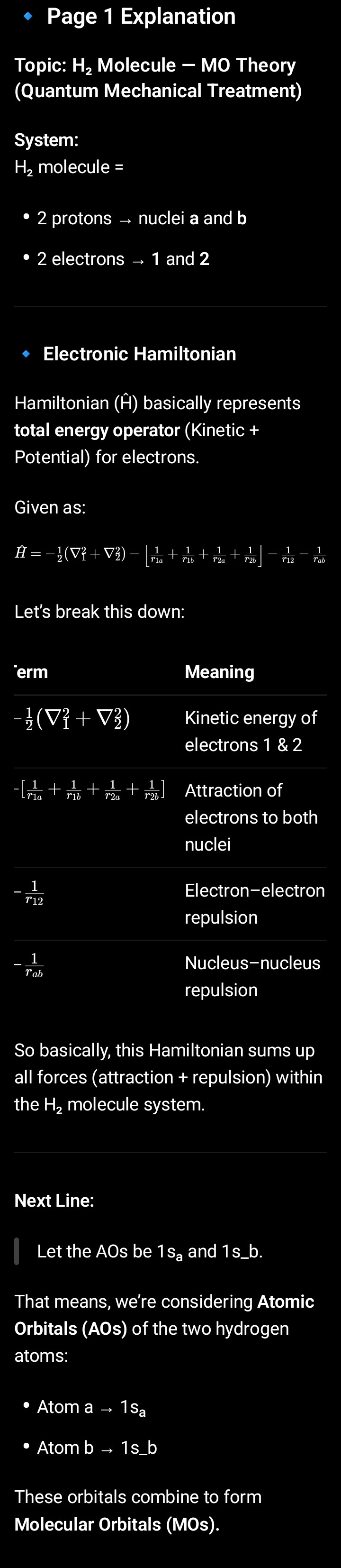
H2(MOT) pg-1
H2(MOT) pg-2

H2(MOT) pg-3
1. Why Schrödinger Equation for H₂ Cannot Be Solved Exactly
The equation is:
\hat{H}\Psi = E\Psi
👉 Problem:1/r12
Dono electrons ek-dusre ke motion ko affect karte hain, isliye inka position variable independent nahi hota.
Matlab — agar ek electron move kare, toh doosre ka potential energy field bhi badal jaata hai.
Isi wajah se, exact solution possible nahi hota.

H2(MOT) pg-4
4. Dissociation Energy of H₂ (Approximation Result)
Using this MO wavefunction, calculated dissociation energy of H₂ = 258.5 kJ/mol
(Experimental = 458.3 kJ/mol)
at an equilibrium bond length ≈ 0.074 nm and internuclear distance 0.085 nm.
👉 So the theoretical (MOT) value = ~56% of the observed value.
Reason for difference: Because this simple MO model ignores:
Electron correlation (interaction between electrons’ motions)
Uses effective nuclear charge instead of actual nuclear charges.
---
⚙ 5. Key Insight
Better results can be obtained if:
We include correlation between electrons, i.e., how one electron’s position affects the other.
And use a more realistic effective nuclear charge (since real protons partially shield each other’s pull).