Pharmaceutics inhaled route - devices
1/79
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
What are the three types of inhalor?
dry powder inhaler, nebuliser, pressured meatered dose inhaler
How are dry powdered inhalers delivered to the lungs (overveiw)?
dry powdered blend, breath-activated, fluidization occurs, enters airway and seporates from carrier particles
What does breath-activated mean?
mechanism activated by the patient breathing in, the patient’s inspiritory effort provides the energy for deaggregation of the formulation, allows small particles deeper into the lungs, get into lower airway and dissolve within the lung fluid, this then absorbed.
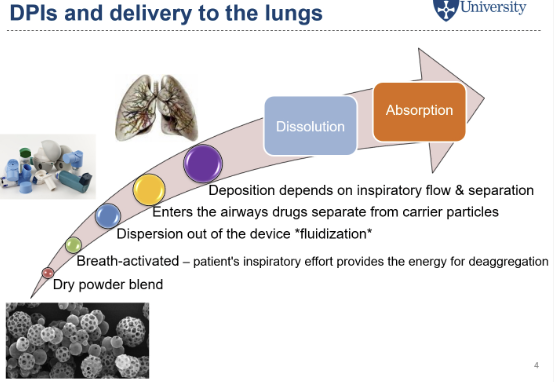
What is fludization?
the force of the patient inhaling through the mouthpeice helps disperse the formulation
What are the advantages of dry powder inhalers?
propellant-free formulations, usually only carrier and API so minimses side effects as less excpents for the immune system to react to, avoids hand-mouth coordination, can deliver larger doses than pMDIs
What are the disadvantages of dry powder inhalers?
powder liberation from divice and particle deaggregation is limited by patient’s abilty to inhale some patients might not be able to do it with enough force, increased air flow increases potential for inertial impaction
What are the main challanges of dry powder inhalers?
need to be 1-5µm for deep lung desposition, these sizes of particles have poor flowability as high cohesive and adhesive, issues with manufacturing, dose uniformity, and reliable aerosolization
Why do you want to be in the specific size range for powder particles for inhalers?
if too small exhaled out, if too big impacted on back of throat
How are drug particles for inhalers normaly made (just name of overall technique)?
they are micoronized to the right size after being synthised in bigger particles
What are the common micoronized techniques?
jet milling, spray-drying, spray-freeze-drying and supercritcal fluid technology
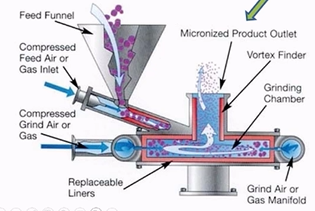
How does jet milling work?
materal fed into the shallow circular grinding chamber
high velocity compressed air is introduced
turbulace causes particles bombarding against each other
centrifugal action the larger particles are concentrated to the periphery of the chamber
finer particles leave with the air stream (1-10µm)
What effects the powder flowablity of particles used in dry power inhalers?
particle size and shape, desity, surface roughness, hardness, moisture content, bulk density
what excpients are used in dry power inhalers, why not any others?
larger carrier substances (30-150µm), choice is limited due to limited buffering capacity of lungs so very few are licenced for the lungs
How do carrier substances work for dry powder inhalers?
drug is mixed with carrier particles producing an ordered mix
drugs attach to larger carrier by weak electrostatic force
decreases drug cohesiveness improving powder flow, aiding liberation of drug from device, improves uniformity for filling so overall uniformity
when inhaled tubulent airflow will deaggregate drug from carrier
free drug penetrates peripheral airways, carrier impacts in throat and remains in mouth so patient can feel power left in mouth even with correct technique, swallowed or rinse mouth
What is the most common large carrier substance for dry powder inhalers?
lactose
What factors effect the formulation design of the carrier?
adhesion and detachment of drug depends on morphology of particle surfaces and surface energies, need balance between blend stability and drug release as inhalation needs to release drug
What are the key parameters of product efficency for carrer molecules used in dry powder inhalers?
drug particle dispersion and size distribution
What are the two catorgaries of carrers?
rough surface carrier and smooth surface carrier
What is the solution for neither rough nor smooth carriers working effectively enough?
use a large rough carrier (lactose) pre-blended with microized carrier particles, fine carrier particles occupy carrer’s crevices, creates smoother surface for drug, now free to detach during inhalation
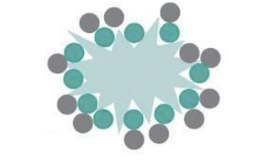
What are the problems with rough surface carrier?
microonized drug gets trapped too strongly leads to too strong adhesion reducing inhaled dose, more stable, allows stuff to flow better

What are the problems with smooth surface carrier?
drug-carrier interactions are minimised so easy deaggregation, the blend may be unstable as the drug can detach during storage, helps with flowability

How do you decide what device to use for a formulation (DPIs)?
need to balance device resistance with patient’s inspiritory flow rate
How do DPI devices work?
patient inhales creating a pressur edrop across the device which provides energy gor powder fluidization and deaggregation
What are the two different DPI devices?
low-resistance devices - require higher IFR to be effective
high-resistance devices - able to generate effective pressure drop with lower IFR, benfical for those with comprimised lung function
What is IFR?
inspiratory flow rate
What do you need to consider when formulating DPIs?
drying, micronizing, carrier (how rough), how to release drugs from the carrier, particle porosity, device
What are the types of DPI devices?
unit dose - capsules, single dose everytime
multi dose reservoir - doses are all in one canister
multi dose pre-packed - blister packs inside the inhalor
How do unit dose inhalors work?
single dose per capsule, placed into device prior to use, device pierces capsule, onhaled airflow through device causes rotor to rotate rapidly, powder is despersed out of capsule into air through porforations, breathing in triggers dose release
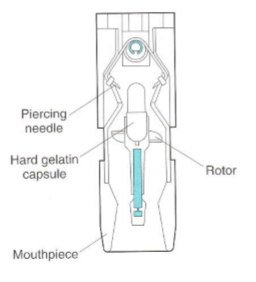
What are some extra features of unit-dose devices?
dust caps to keep mouthpeices clean,
How do multi-dose devices?
diskhalers - drug/lactose mix is filled into bliser with x doses preloaded into the device , each dose is packaged seporatley so only exposed to ambient conditions prior to doseing
turbohaler etc. - x doses of undiluted loosely aggregated mcroionised drug in a reservoir, drug flows on to a rotating disc in the dosing unit with excess removed by scrapers as the rotating disk is turned, requires higher IFR due to higher internal resistance, more sensitive to humidity than accuhalor (diskhalor)
How do pMDIs work?
drug is dissolved or suspended in liquidified propellent mix inside the propellant canister, the different atmospheric pressures maintain it in a liquid
actuation - pressing the canister releases a metered volume through a narrow orrifice in the actuator
atomisation - the sudden pressure drop causes the propellant to atomise/evaporate, drug is propelled as a high-speed spray of fine droplets into the airway using momentum
What are the 5 key componants of pMDIs?
actuator, container, metering valve, propellant, formulation
What do you need to know about pMDI containers?
aluminium genrally as chemically inert with most drug formulation content, maintains pressure, 10-30mL (if too big
What do you need to know about metered valves in pMDIs?
ensures reproducable dose (25-100µL) is delivered each time, used in inverted position, valve stem fits into actuator, it is a spring which is pressed down which triggers the metering valve to release the drug, if unused (in long time or at all) the metering chamber may be empty or contain air so inhalor needs to be primed actuate the device to waste to fill up the metering chamber, the valve stem fits into the actuator
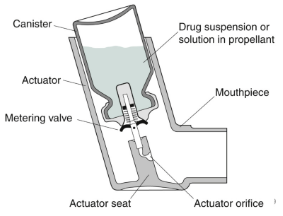
How does the metering valve work in pMDIs?
metering chamber is filled from bulk canister at resting stage, during actuation depression of the valve stem empties the chamber’s contents through the stem orifice, after actuation the release in pressure allows the metering chamber to refill with liquid from the bulk for the next dose
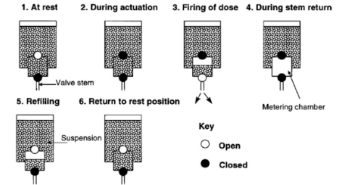
What do you need to know about the actuator in pMDIs?
polyethylene or polypropylene, dimentios of orifice and the propellant vapour pressure determine the shape and speed of emitted aerosol cloud so determined theraputic doses
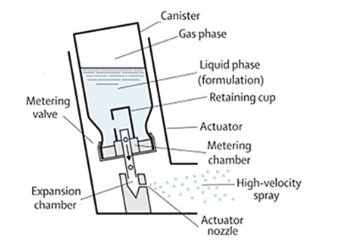
What are propellants?
liquidified gas needed for pressurisation and atomisation, maintained as liquid under high pressure in canister but gasses at room temp and pressure, provide needed pressure to atomise drug formulation into micron-scaled droplets when accuated
What propellants are used in pMDIs?
hydrofluoroalkanes (HFA-134a and HFA-227) (used to be chlorofluorocarbons but they are ozone depleting)
What are the disadvantages of HFAs?
poor solvents so excipients often used increasing side effects, potent greenhouse gasses contibuting to climate change , active search for next generation propellant but complex process
Are there more pMDIs that are solutions or suspensions? why?
suspentions as HFAs are poor solvents
What do you to consider when formulating a pMDI?
drug solubility in propellant, densities of propellant, particle size, valve clogging, moisture content, drug stability
What are the challanges for pMDI solutions?
drug dissolved in propellant, liquid after it has been liberated
What are the challanges of pMDIs suspentions?
solid drug disspersted in propellent, when its actuated, liquid after dispersed (?)
How do you solve the problem of low solubilities in HFAs in pMDI solutions?
use cosolvents such as ethanol (small amounts so no toxicity effect)
How do you solve the problem of physical stability in pMDI suspensions?
needs surfactants such as oleic acid to prevent aggregation and caking
What is aerosol generation like for pMDI solutions?
propellant evaporation forms low-mass “droplet reminants”, smaller particle size means greater lung desposition
What is aerosol generation like for pMDI suspentions?
propellant evaporation liberates the original micronized drug particles, larger paticle size means more oropharyngeal desposition, may need to increase dose
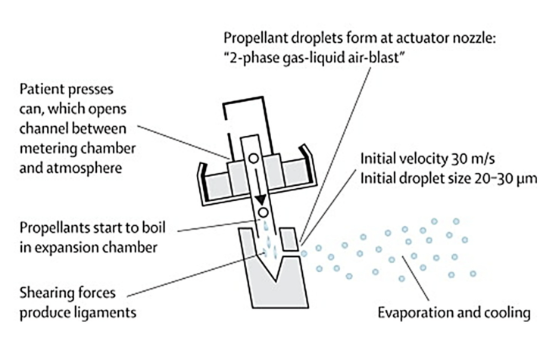
How is an aerosol generated from a pMDI?
patient presses can opening channel between metering chamber at atmosphere, propellants start to boil in expansion chamber (temp also changes), shearing forces produce ligaments, propellant droplets form at actuator nozzle, evaporating and cooling then occurs, drug come out in a stream, front ones have higher volcity and bigger droplet size which are affected by inertial impaction more
What coucelling advice do you give to patients for pMDIs?
14 days or more one puff away to check its working, hold canister vertically with body upwards, breath in slowly and deely, hold your breath for as long as possible, do not breath out into the inhalor
What are the problems with pMDIs?
>30m/s velocity causes oropharyngeal impaction - up to 80% is swallowed and lost, requires coordnation actuation with slow, deeo, inhalation - requires appropriate councelling, chilling sensation from the cold propellant emitted can interfere with the patients ability to inhale properly (cold freon effect - more for older ones), 10-20% of the dose may be delivered to site of action
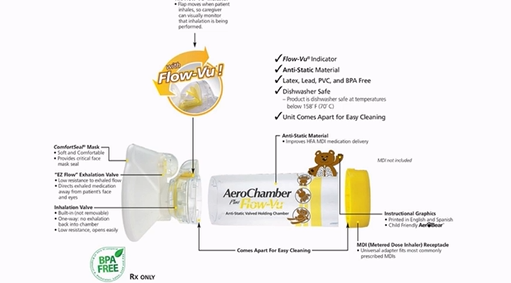
What are spacer devices?
plastic chamber with port at one end for device, a one way valve and mouth peice at the other, they are device specific so ensure compatable, actuator is pressed down and allows patient to take their time to breath in and out slowly, big and cumbersome
How do spacers work?
actuator is pressed down, large droplets impact on the spacer walls, propellant evaporates and velocity of drug droplets reduces, so the aerosol cloud is finer and slower moving compared to directly released, patient can inhale from valve after actuation, reduces oral impaction and improves pulmonary deposition
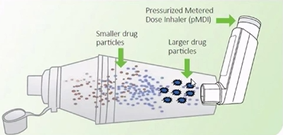
What inhalation aids are there?
a mask can be attached or be integral to spacers which can be placed over the nose and mouth (young patients especially)
What are breath-acuated pMDIs?
patient’s inhalation triggers actuation, using a spring-loaded or vacuum-tiggered mechanisms, avoids the coordination problem without a bulky spacer, requires a sufficient inspiritory flow rate to trigger release

What is a nebuliser?
a device that converts a liquid formulation into a continuous aerosol for inhalation via a face mask, it is effective with normal tidal breathing, no coordination is required and you can deliver higher doses than with standard inhalers
What are the key clinical indications for the use of a nebuliser?
acute exacerbations of athsma and COPD in hospital, regular therapy for patients with severe airway conditions who cannot use pMDIs/DPIs, delivery of specific drugs (cyctic fibrosis), for prophylaxis od pneumocystis pneumonia
How do nebulisers deliver drugs to the airway?
drug is dissolved/suspended in aqueous solvent (usually water) added to nebuliser drug resivoir, nebuliser connected to power source, energy is applied to generate an aerosol, generates high proportion of droplets in 1-5µm range, desposition independent of inspiritory flow rate
How is the type of nebuliser decided?
chosen due to the desposition required and viscosity of solution
What particle size is needed for alveolar despositition?
1-2µm
What does location of deposition for nebulisers depend on?
particle size, pattern of inhalation, and lung disease severity
What are the types of nebulisers?
Jet, ultrasonic, vibrating mesh
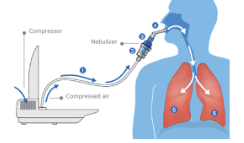
What do nebulisers genrally consist of?
compressor suppling compressed air, nebulizing chamber where drug goes, mouthpeice or face mask
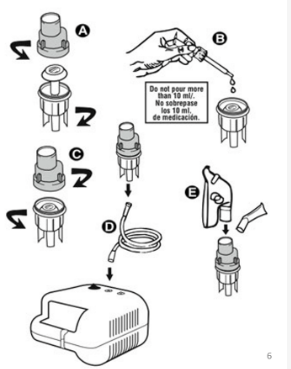
What are Jet nebulisers?
(air-jet or air-blast) most widley used, cheaper than mesh, optimum flow rate of 6-8L/min, uses compressed air or oxygen in hospital or electical compressor at home
What does the flow rate determine?
droplet size and rate of drug delivery, higher flow rate, smaller droplet size and higher velocity
How do jet nebulisers work?
Venturi effect - compressed gas passes through narrow capillary tube, increases air velocity creating -pressure around venturi nozzle
Bernoulli effect - causes drug formulation to draw up a feed tube from liquid resivoir - then fragmented into droplets
large droplets impact on baffles and recycle, small droplets exit via airstream
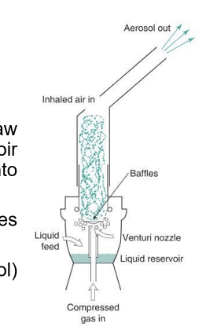
What are the issues with jet nebulisers?
compressers compersome and noisy, operated continously so large proportion of emitted aerosol is released into the air during exhalation - drug waste
What are ultrasonic nebulisers?
a piezoelectric crystal vibrates at high frequency, creates ultrasonic waves causing liquid to form fine mist, needs power source to create waves
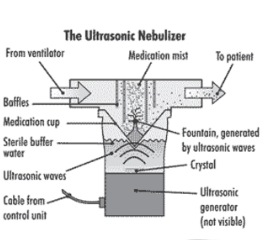
What are the advantages and disadvantages of ultrasonic nebulisers?
more expensive, quieter, no gas flow, not sutible for suspensions
What is a vibrating mesh nebuliser?
aerosols are generated by passing liquid drug through vibrating mesh producing 1-5µm droplets, many times per second
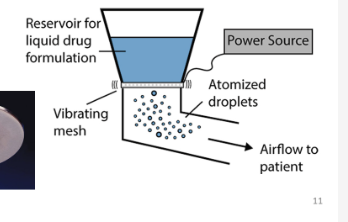
What are the advantages and disadvantages of vibrating mesh nebulisers?
silent, most portable, higher cost, suspensions can block the mesh if not maintained
What should be avoided added to nebuliser fluids?
antioxidants and preservatives as may cause bronchospasm
What are the pH requirements of nebuliser fluid?
between 3-10 to avoid iritation
What should you use if needing to dilute nebuliser fluid?
preservative-free 0.9% saline
What do nebuliser fluids normally contain?
mostly preservative-free sterile water mostly as solutions, occationally a co-solvent is added, surfactants may be added, sterile unit doses without preservatives to prevent contamination, must be iso-osmotic to avoid brochoconstriction
How does tempreture effect Jet nebulisers?
temp in fluid will drop 10-15°C due to evaporation of solvent, could effect asthma patients which bronoconstriction on inhalation of cold solutions, drug solubility may be reduced due to colder temp result in increased surface tension and viscosity
How does tempreture effect ultrasonic nebulisers?
increase in fluid temp by 10-15°C, more suitible for less aqueous soluble drugs, not suitible for heat sensitive drugs
What is residual volume?
not matter the duration of nebulisation, not all fluid in nebuliser can be atomised, affects dose patient receives, using wrong nebuliser for specific drug can cause under-dosing
What are the residual volumes of the different types of nebulisers?
Jet/Ultrasonic - high ∼1mL, vibrating mesh - very low residual volume
Explain device variability
different nebuliser models and compressor can produce very different aerosol sice and delivered dose, also between nebulisers of same type, baffle wear and tear and non-uniformity of assembly also causes variability, most solutions are not produced by the same company as make the nebulisers
What are the patient counselling points for nebulisers?
follow device specific guidelines, avoid mixing drugs unless advised by HCP - if needed mix immediatly before use and discard if turbid, if needs dilution use 0.9% preservative-free saline, clean after every use with mild detergent and allow to air dry to prevent microbial contamination and clogging, instruct patient not to treat acute attacks without seeking medical help