MED CHEM QUIZ 7 💊
1/175
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
176 Terms
Types of Lipids
Triglycerides – energy storage
Phospholipids – form cell membranes
Cholesterol – used to make hormones, bile acids, membranes

Cholesterol
Cholesterol and Triglyceride Properties
Highly lipophilic
High logP
Poor water solubility
Found in high proportions in the blood through lipoproteins
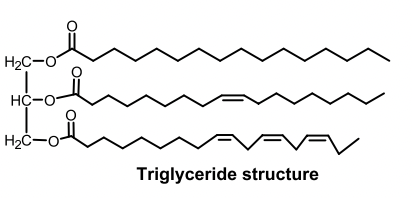
Triglycerides vs Phospholipids
Phospholipids: Have an additional phosphate and choline molecule

Triglyceride
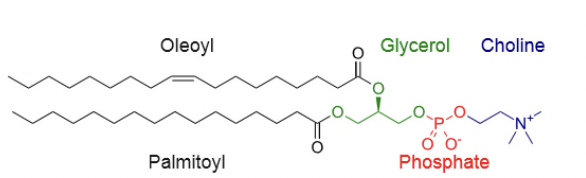
Phospholipid
Lipoproteins
Polar macromolecular lipid-phospholipid protein spherical assembly that is water soluble.
They consist of a lipid monolayer with polar surface exposed to water and hydrophobic tails pointing inward.
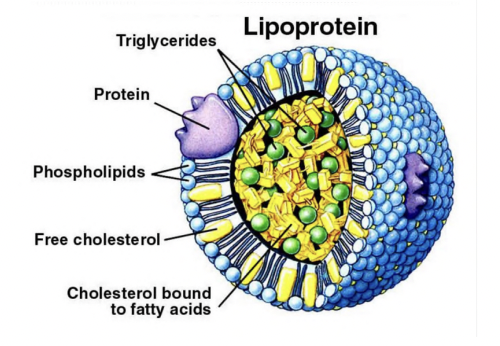
Apolipoproteins
Embedded in the phospholipid monolayer of lipoproteins.
They provide:
Structural stability
Recognize and bind to specific receptors
Act as cofactors for the enzymes that metabolize lipoproteins.
CC Lipoproteins Size & Density
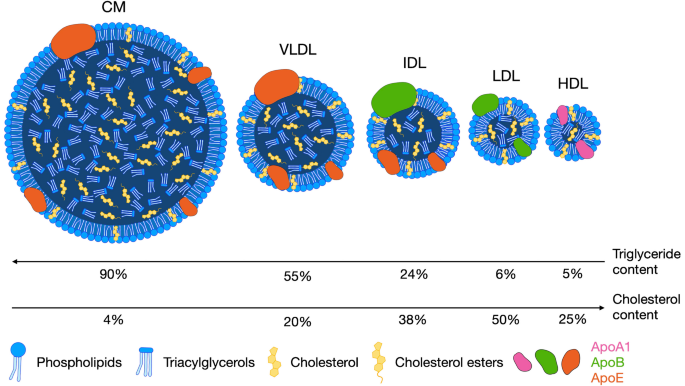
Density ↑ and size ↓ from chylomicrons → HDL
Protein content ↑, triglyceride ↓ along this order.
Lipoprotein with the most cholesterol/cholesterol esters (CE)?
LDL
Identify the correct statement about Apolipoproteins?
A. They have a lipid bilayer
B. All of them have the same apoplipoteins
C. Apoplipoproteins give them specificity to bind to cellular receptors
D. Chylomicron is not a lipoprotein
E. HDL has the highest triglyceride count
C. Apoplipoproteins give them specificity to bind to cellular receptors
Apolipoproteins
Provide structure and receptor binding.
Act as enzyme cofactors (e.g., ApoC-II activates lipoprotein lipase).
Examples:
ApoB-100: Needed for LDL receptor binding
ApoC-II: Activates lipoprotein lipase
ApoE: Helps clear chylomicrons and VLDL remnants
Hyperlipidemia / Dyslipidemia
Hyperlipidemia = high levels of lipids/lipoproteins in blood.
Dyslipidemia = abnormal lipid levels (↑LDL, ↑TG, ↓HDL).
Primary causes of Hyperlipidemia
↓LDL receptors → ↑LDL
Defective ApoB-100 → ↑LDL
ApoC-II deficiency → ↑TG (chylomicrons, VLDL)
Defective ApoE → ↑VLDL, IDL (mixed high TG + cholesterol)
How do low LDL receptors cause primary hyperlipideima?
LDL-rs uptake LDL from the blood back to the liver.
Fewer receptors → more buildup of LDL
Secondary Causes of Hyperlipidemia
Diabetes, hypothyroidism, liver/renal disease.
How does low (or defective) apoB100 lead to primary hyperlipideima?
ApopB-100 recognition is needed for the uptake of LDL by LDL-r.
How does ApoC-11 deficiency lead to hyperlipidemia?
ApoC-II is recognized by lipoprotein lipase, the enzyme that catalyzes chylomicron.
ApoC-II deficiency → lipoprotein lipase inactive → ↑ TG
How does ApoE deficiency lead to hyperlipidemia?
Poor clearance of VLDL/chylomicrons → ↑ TG + cholesterol
Primary vs Secondary Hyperlipidemia
Primary=Genetics
Secondary=Disease
Familial hypercholesterolemia
Hypercholesterolemia caused by LDL receptor deficiency
Familial defective ApoB-100
Hypercholesterolemia caused by Mutant ApoB-100
Hypertriglyceridemia Example
Familial apoC-II deficiency
Mixed hyperlipidemia Example (Hypercholesterolemia + Hypertriglyceridemia)
Mixed hyperlipidemia due to a defective apoE
Mixed Hyperlipidemia (high cholesterol and high triglycerides), elevated plasma triglycerides can lead to what?
Coronary Heart Disease
Pure hypertriglyceridemia
Associated with pancreatitis
Cause: ApoC-II Deficiency, which is needed for the recognition of chylomicrons by lipase.
Lipase cleaves triglycerides to DAG and free fatty acid (FFA).
Which lipoproteins are the largest and have the largest TG content?
Chylomicrons
VLDL
Apo C-II on them catalyzes the activation of lipase for the breakdown of them.
Deficiency in this → Pure hypertriglyceridemia
Major Sources of Liver Cholesterol
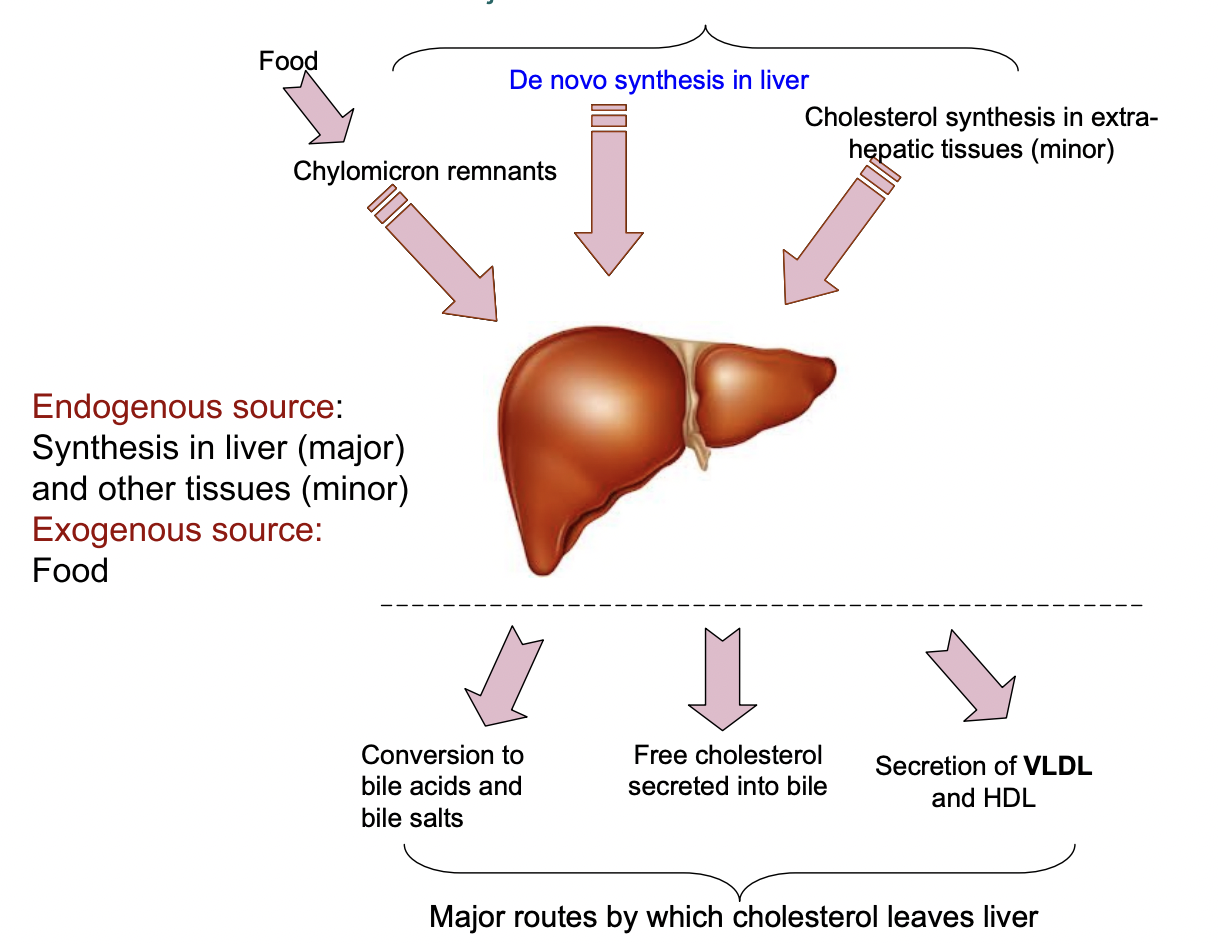
Sources of Cholestrol
From diet (chylomicrons)
Made in liver (de novo synthesis)
Minor synthesis in extrahepatic tissues
Fate of cholesterol
Used for bile acid synthesis
Secreted into bile
Used in membranes or hormone synthesis
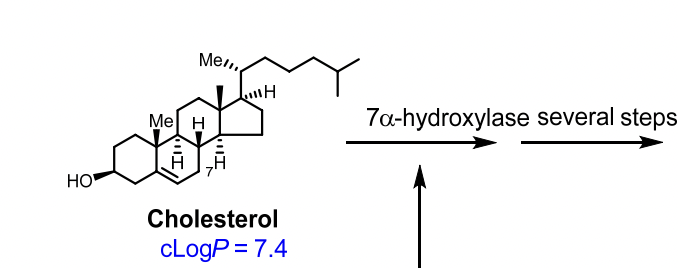
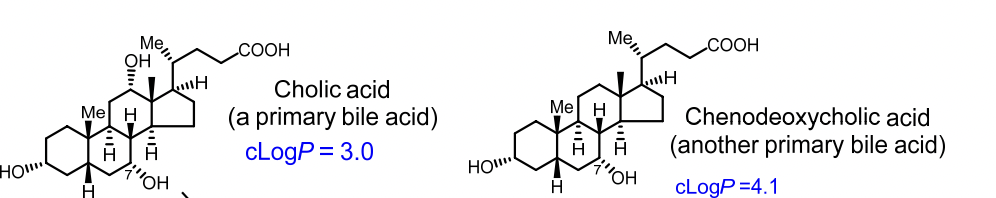
Primary Bile Acids
Primary vs Secondary Bile Acids
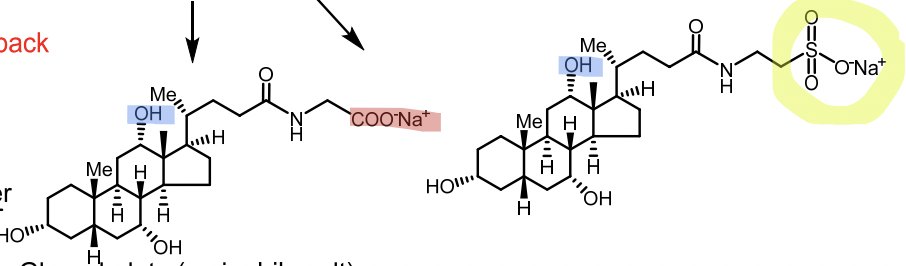
Primary bile acids: Cholic acid, Chenodeoxycholic acid
Conjugated with glycine or taurine (secondary) → glycocholic acid, taurocholic acid
Bile salts
Ionized (active) form of conjugated bile acids at pH 7.4
Glycocholate
Taurocholate
Bile Salts MOA
Emulsify fats for digestion and absorption of lipids & fat-soluble vitamins
Enterohepatic circulation
Bile salts secreted → intestines → reabsorbed → portal vein → liver
High bile salt concentration → inhibits 7α-hydroxylase → stops further bile acid synthesis (negative feedback)
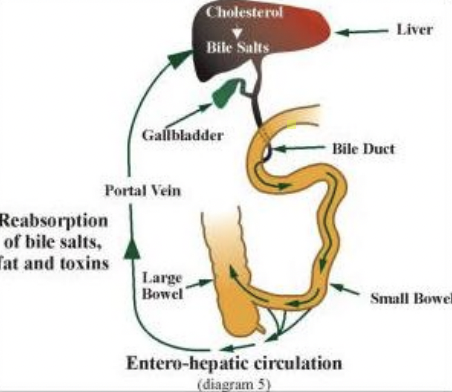
Primary Bile Acids are formed how?
Cholesterol + 7α-hydroxylase → Cholic Acid or Chenodeoxycholic Acid
Bile Salts
Glycocholate
Taurocholate
Salt=Secondary
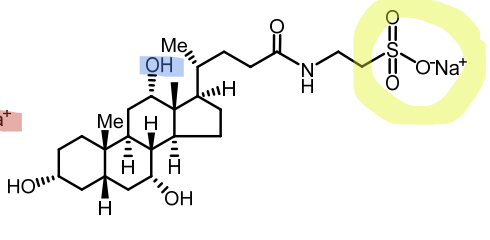
Taurocholate
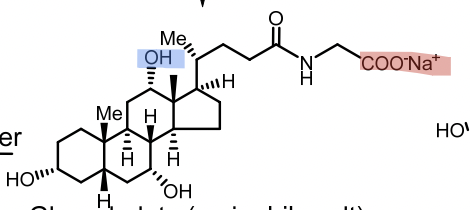
Glycocholate
Taurocholate vs Glycocholate
Triglyceride Metabolism
Made from glycerol-3-phosphate + fatty acyl-CoA
Stored in adipose tissue
Broken down by lipase → free fatty acids (FFA) for energy
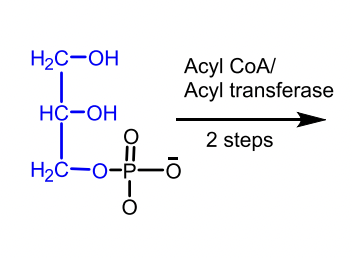
Glycerol 3-phosphate

Why are triglycerides energy-rich?
Because oxidation of fatty acids releases a large amount of ATP.
What are lipids? What are the three classes of lipids in the body?
Lipids are water-insoluble, fat-soluble molecules important for energy storage, membranes, and signaling.
Three main classes:
Triglycerides – energy storage form in adipose tissue
Phospholipids – make up cell membranes
Cholesterol – used for hormones, bile acids, and membranes
Be able to identify the three classes of lipids structurally.
Triglyceride: glycerol backbone + 3 fatty acids
Phospholipid: glycerol + 2 fatty acids + phosphate group (often with choline)
Cholesterol: four fused hydrocarbon rings (steroid nucleus) + hydroxyl group
What are the solubility characteristics of lipids?
Lipids are nonpolar and hydrophobic → they do not dissolve in water.
They dissolve in nonpolar solvents (like ether or chloroform).
Because of this, lipids must travel in blood as lipoproteins.
What are the functional roles of different lipids?
Lipid Type | Function |
|---|---|
Triglycerides | Store energy in adipose tissue |
Phospholipids | Form cell membranes and lipid bilayers |
Cholesterol | Maintains membrane fluidity, forms steroid hormones and bile acids |
What is one major advantage of lipoproteins in an aqueous environment compared to lipids?
Lipoproteins make insoluble lipids transportable in blood (which is mostly water).
→ They have a hydrophilic outer layer (proteins + phospholipids) and hydrophobic lipid core.
How does logP help predict aqueous solubility?
LogP = partition coefficient = ratio of solubility in octanol (fat) vs. water.
High logP (>1): more lipid-soluble, less water-soluble
Low logP (<1): more water-soluble
➡ Example: Cholesterol has a high logP, so it’s very lipid-soluble.
Define dyslipidemia and hyperlipoproteinemia.
Hyperlipoproteinemia: Too many lipoproteins or lipids in the blood (LDL, VLDL, TG).
Dyslipidemia: Abnormal lipid pattern → ↑LDL, ↑VLDL, ↑TG, ↓HDL.
(Dyslipidemia is more precise because it includes both high and low abnormal levels.)
How are bile acids formed? What is the major enzyme involved? What are the major bile acids?
Formed in liver from cholesterol.
Enzyme: 7α-hydroxylase (rate-limiting step).
Major bile acids: Cholic acid and Chenodeoxycholic acid.
What is enterohepatic circulation?
Recycling pathway of bile salts:
Bile salts made in liver → secreted into intestine → help digest fats
Most reabsorbed in ileum → return to liver via portal vein
High bile salt levels → inhibit 7α-hydroxylase (negative feedback on cholesterol → bile acid conversion)
What are the functional roles of apolipoproteins?
Provide structure to lipoproteins
Bind to receptors for lipoprotein uptake (e.g., ApoB-100 for LDL receptor)
Activate enzymes for lipid metabolism (e.g., ApoC-II activates lipoprotein lipase)
Reverse cholesterol transport (HDL)
HDL picks up cholesterol from tissues → liver
Why is HDL called “good cholesterol”?
HDL removes cholesterol from tissues and arteries → brings it back to liver for excretion (reverse cholesterol transport).
➡ Prevents plaque buildup and protects against heart disease.
What is reverse cholesterol transport (RCT)? Why is it beneficial?
RCT: Process where HDL collects cholesterol from peripheral tissues → converts it to cholesterol esters via LCAT → delivers it to the liver.
Benefit: Clears excess cholesterol → reduces atherosclerosis risk.
Know the role for LDL receptors.
LDL receptors on the liver bind LDL via ApoB-100 → remove LDL cholesterol from blood.
Low receptor number or defective receptor = high LDL in blood → familial hypercholesterolemia.
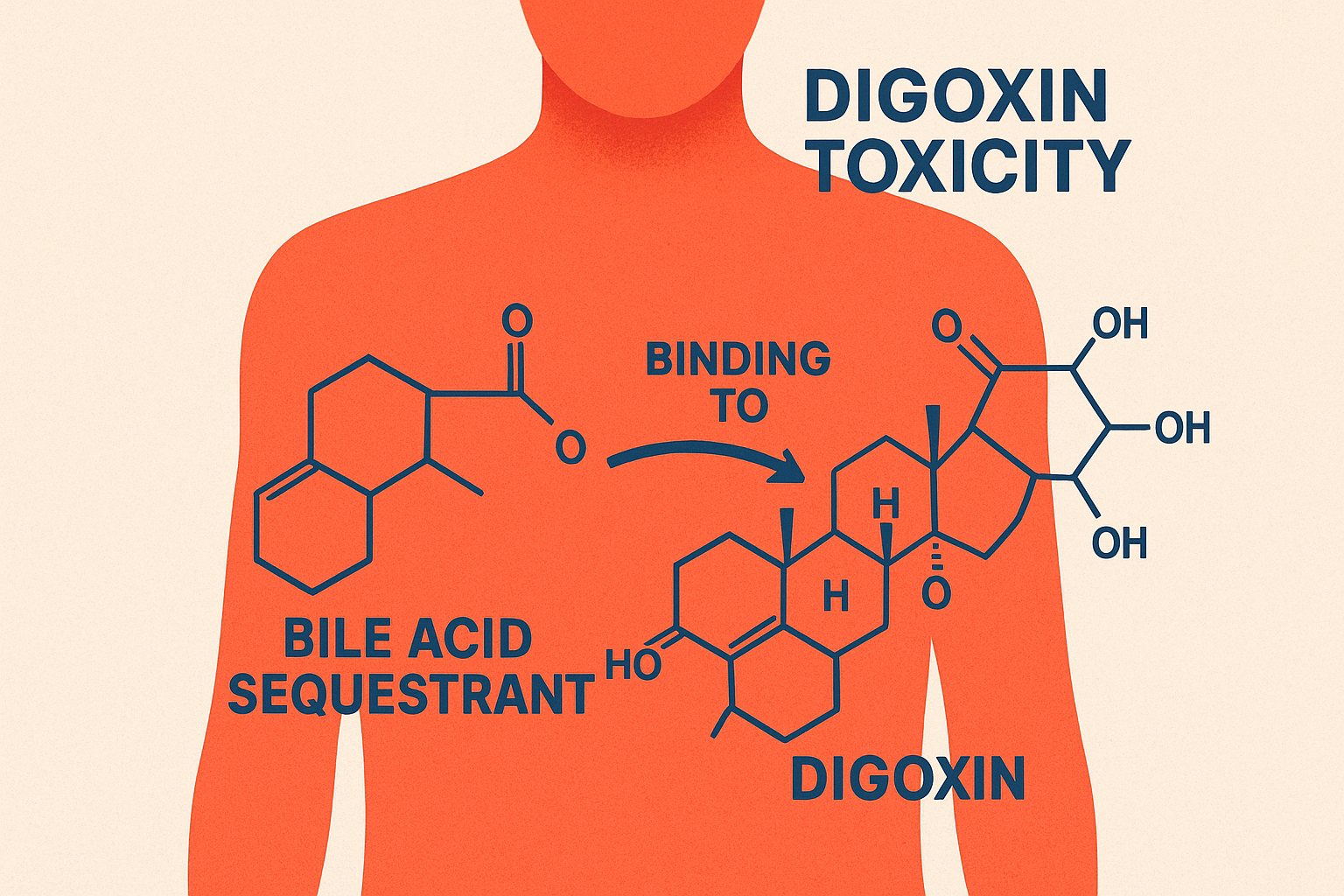
What is the mechanism of action of bile acid sequestrants?
They bind bile acids in the intestine and form an insoluble complex that gets excreted in feces.
→ This prevents bile acid reabsorption into the liver.
How do bile acid sequestrants lower liver cholesterol and blood LDL?
When bile acids are lost, the liver needs to make more → it uses cholesterol from the blood.
This lowers liver cholesterol, which causes the liver to increase LDL receptors → more LDL removed from blood → lower LDL levels.
How do bile acid sequestrants cause an increase in liver HMG-CoA reductase activity?
The liver senses low cholesterol (because it’s being used to make bile acids),
so it activates HMG-CoA reductase to make more cholesterol.
What drugs are bile acid sequestrants usually combined with to reduce LDL cholesterol?
They are often combined with statins or ezetimibe for a stronger LDL-lowering effect.
Why are bile acid sequestrants called anion exchange resins? What anions are exchanged?
They exchange chloride (Cl⁻) ions for bile acids (anions) in the intestine.
→ The bile acids bind to the resin and get excreted instead of being reabsorbed.
Bile Acid Sequestrants
MOA: Prevent the reabsorption of bile acids in the liver. The liver then uses cholesterol to form more bile and also expresses more LDL-receptors.
They also exchange chloride ions (Cl⁻) for bile acid anions in the intestine.
Effects: ↓ LDL cholesterol

Uses for bile acid sequestrants.
Primary hypercholesterolemia (high LDL from genetic causes)
Adjunct to statins to further lower LDL
Pruritus in cholestasis (itching from bile acid buildup)
Digitalis (digoxin) toxicity – binds and removes it from intestine
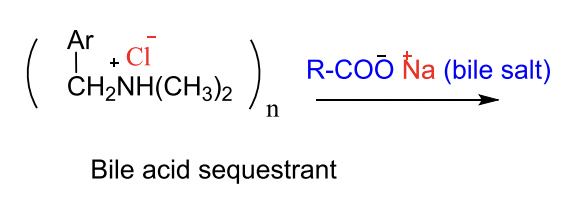
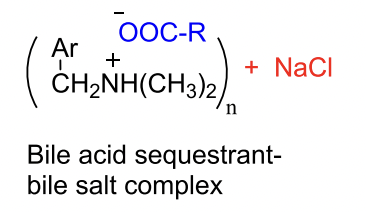
MOA of BIle Acid Sequerants
How does the body compensate if you give a patient bile acid sequestrants?
Increased expression of HMG-CoA reductase (slow process) and increase in triglyceride/VLDL synthesis in the liver.
Are bile acid sequestrants used for patients with familal hypercholestermia?
No. Genetic deficient in the number of LDL receptors and this drug doesn’t cause a major change in LDL-receptor expression.
Indication for Bile Acid Sequestrants
Hypercholesterolemia in patients who don’t adequately response to dietary modifications in combo with HMGRIs (statins) or niacin.

Cholestrylamine
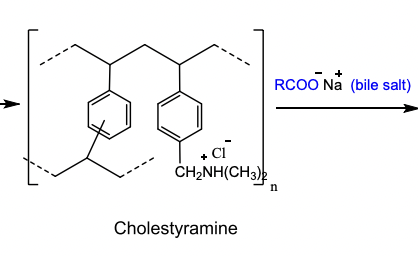

MOA of Bile Acid Sequestrants → exchange the anion for the ble acid
Why is the Cl- replaced by RCOO- by bile acid sequestrants?
The R group of bile acid has stabilizing secondary interactions making the backbone of bile acid sequestrants more stable.
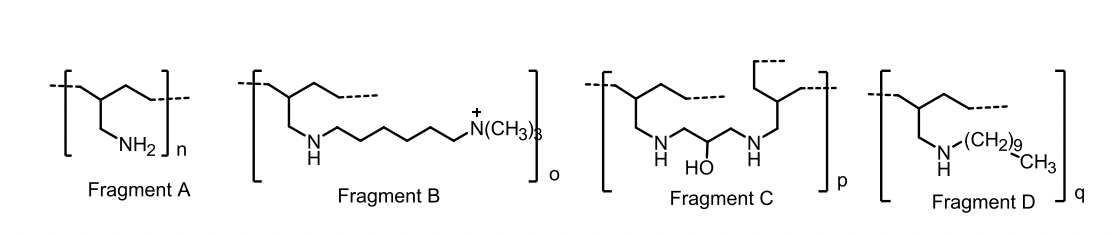
Cholestyramine is a copolymer of what?
Styrene and divinylbenzene, containing quaternary ammonium functional groups that bind bile acids through ionic attraction.
These positive nitrogen groups exchange Cl⁻ for bile acids in the intestine.
Cholestyramine
Powder that is mixed with non-carbonated beverages as a slurry to drink.
Daily dose: 8-16 grams/day
Indication: Primary Hypercholesterolemia (elevated LDL cholesterol) and pruritus.
Warning: Chronic Use → Increases the bleeding risk due to hypoprothrombinemia.
Forms of Bile Acid Sequestrants
Cholestyramine → powder
Colestipol, Colesevelam → tablets
Colestipol MOA
Copolymer of tetraethylenepentamine and epichlorohydrin.
Basic secondary and tertiary amine groups on colestipol are protonated at intestinal pH and bind to bile acids.
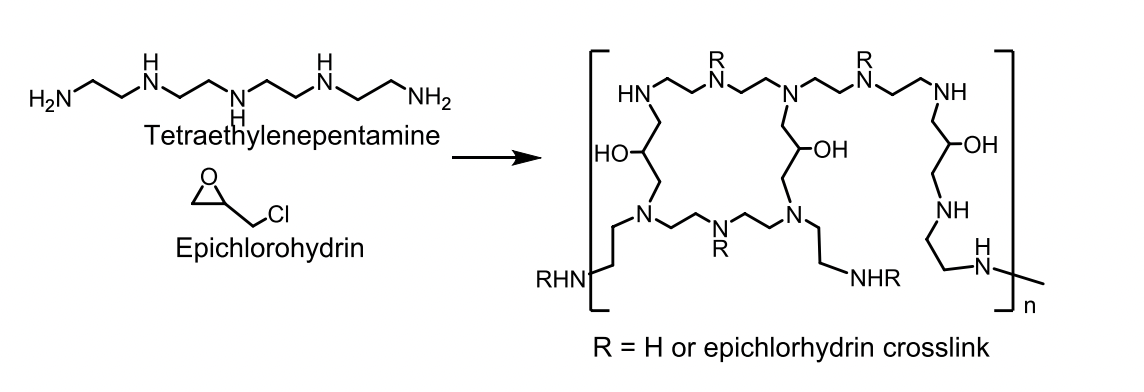
Colestipol
Granules or 1-gram tablets
Dose: 5-30 g/day for granules
Indicated for primary cholesterolemia and also hyperglycemia
Warning: Other drugs should be administered at least 1 hour before or 4 hours after colestipol.
Colesevelam
A mixture of several polymeric compounds.
Available as a tablet or powder for suspension.
Indication:
Hypercholesterolemia and glycemic control in Type 2 diabetes (since they decrease the absorption of nutrients).
Warning:
(For all bile acid sequestrants): Decreases the absorption of Vitamin A, D, E, K, and other vitamins/drugs should be taken within 4 hours of colesevelam.
Colesevelam
What are the most common adverse effects of bile acid sequestrants?
Constipation, bloating, nausea, abdominal discomfort, and GI irritation.
These can be reduced by increasing fluids or taking with meals.
What vitamins and nutrients are affected by bile acid sequestrants?
They decrease absorption of fat-soluble vitamins A, D, E, and K, which may lead to hypoprothrombinemia (vitamin K deficiency).
Vitamins should be taken at least 4 hours before Colesevelam or other bile acid sequestrants.
What are key pharmacokinetic features of bile acid sequestrants?
They are not absorbed orally, have no hepatic metabolism, no CYP450 interactions, and are excreted unchanged in feces.
The onset is 24–48 hours and full therapeutic effect takes up to a month.
What serious adverse effect can occur with long-term bile acid sequestrant use?
Hypoprothrombinemia (bleeding) due to vitamin K deficiency.
Bleeding from Bile Acid Sequestrants
What are important drug–drug interactions with bile acid sequestrants?
They can decrease absorption of several drugs: tetracycline, phenobarbital, digoxin, statins, fibrates, and warfarin.
Other drugs should be taken 1 hour before or 4 hours after to avoid binding in the intestine.
Do bile acid sequestrants affect liver enzyme metabolism (CYP450)?
No, they are not absorbed and do not affect CYP450 enzymes.
What condition is a contraindication for bile acid sequestrants?
Primary biliary cirrhosis (they can raise hepatic cholesterol and worsen the condition).
What are the unlabeled (off-label) uses of bile acid sequestrants?
Treatment of digitalis (digoxin) toxicity by binding digoxin in the gut, hyperthyroidism by binding thyroid hormones, and antibiotic-associated colitis or C. difficile diarrhea by binding bacterial toxins.
Why are bile acid sequestrants considered among the safest lipid-lowering drugs?
They are not absorbed into systemic circulation, have no hepatic metabolism, and do not cause systemic toxicity.
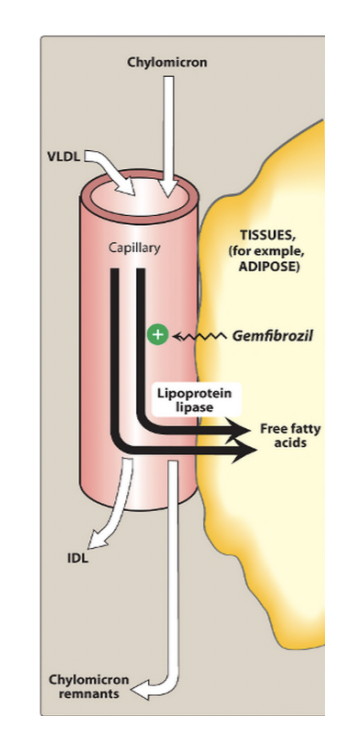
What are fibrates and their main lipid effects?
Fibrates are lipid-lowering drugs that activate PPAR-α receptors to increase lipoprotein lipase (LPL) activity, leading to breakdown of triglyceride-rich particles like VLDL and chylomicrons.
This causes ↓ triglycerides, ↓ VLDL, and ↑ HDL. They are mainly used for hypertriglyceridemia and mixed dyslipidemia.
What receptor do fibrates activate and what does it do?
Fbrates activate PPAR-α (Peroxisome Proliferator-Activated Receptor Alpha), which regulates genes controlling lipid metabolism.
Activation decreases ApoC-III (an inhibitor of LPL) and increases fatty acid oxidation and HDL production (via ApoA-I and ApoA-II).
How were fibrates founded?
Identified from random screening of a series of aryloxylisobutrytic acids.
Clofibrate was the first compound to be marketed for its discovery efforts.
No evidence that fibrates decrease cardiovascular outcomes.
What are the main examples of fibrate drugs?
Gemfibrozil, Fenofibrate, and the older Clofibrate (discontinued).
Triglyceride Effects of Fibrates
Remove triglycerides significantly
Promote degradation by stimulating the activity of lipoprotein lipase
Inhibit the synthesis of TG
SIGNIFICANTLY REDUCE VLDL
Modest cholesterol reduction → This, however, increases the risk of gallstone production.
Increase HDL through transcription of apoA-I and apoA-II.
Approved Use of Fibrates
Hypertriglyceridemia and familal combined hyperlipidemia
Can be used in combination with niacine, bile acid sequestrants or HMGRIs (statins) but then there is an increased risk of rhabdomylsis
If used with bile acid sequestrants → dosing should be spaced at least one hour apart
SAR for Fibrates
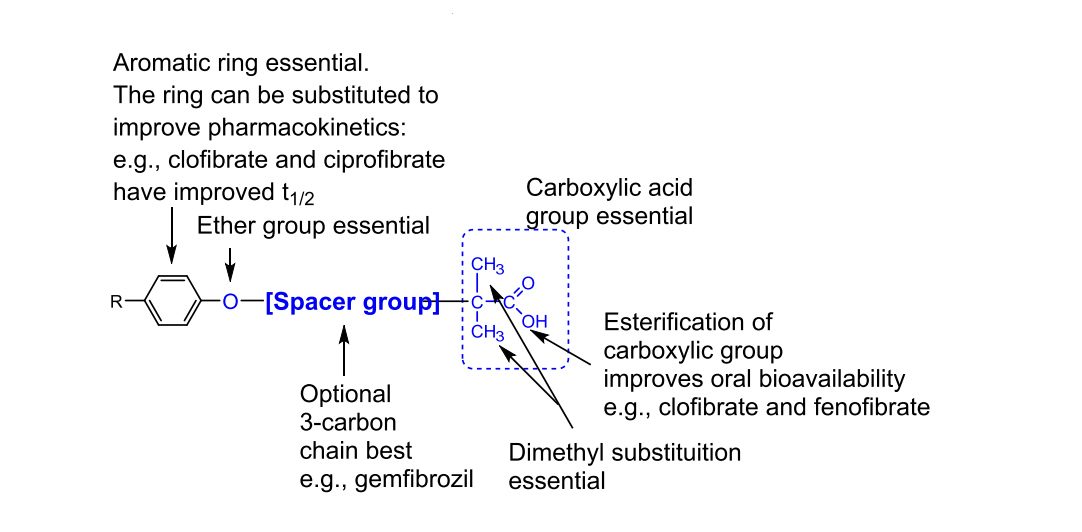
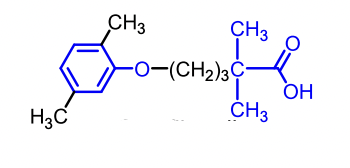
Gemfibrozil
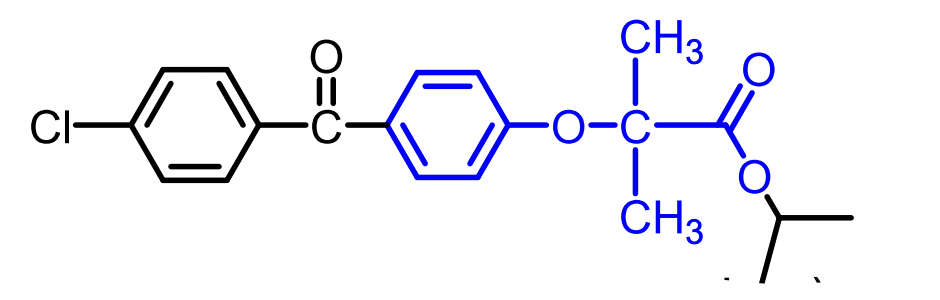
Fenofibrate (ester prodrug)
DDIs for Fibrates
HMGRIs (Statins) → Increased risk of severe myopathy or rhabdomyolysis
Oral Anticoagulants → Increased risk of hypoprothrombinemia
Ezetimibe → Higher blood concentration of Ezetimbe → Possible Cholethiasis
ADRs for Fibrates
Common ADR: GI Toxic
Can cause rhabdomyosis (like statins) this effect is increased when in combo
Can cause elevation in liver enzymes (AST, ALT, Creatinine Phosphokinase).
Gemfibrizol and Fenofibrate are less problematic than Clofibrate
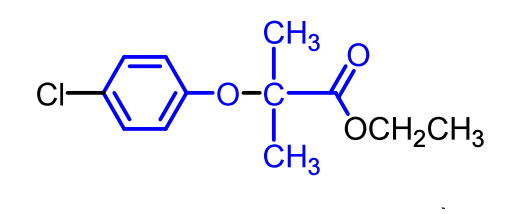
Clofibrate