HCR 240: Final Exam 💯
1/271
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
272 Terms
what is hypoxia (mechanism of cell injury)
- lack of oxygen in cell/tissue
- can be due to lack of oxygen in air, lack of hemoglobin/hemoglobin function, reduced number of rbc, respiratory/cardiovascular diseases, but most common is ischemia (lack of blood supply)
what happens if oxygen is restored in hypoxia (mechanism of cell injury)
- ischemia-reperfusion injury can occur (secondary injury that results due to generation of reactive oxygen intermediates (free radicals) within mitochondria)
- Mitochondria responds by opening mitochondrial permeability transport pore, which lead to massive escape of ATP and solutes, triggering cell death by apoptosis
what happens when a cell loses oxygen (mechanism of cell injury)
- decreased ATP production in mitochondria
- failure of active transport mechanisms in cell membrane (na/K pumps)
- inability to regulate osmosis (cellular swelling)
- detachment of ribosomes from ER
- lysis of plasma membrane
- cell death.
what is asphyxiation → suffocation, strungulation, chemical, and drowing (mechanism of cell injury)
- asphyxiation: injury due to failure of cells to receive or use oxygen
- Suffocation: lack of oxygen in environment or blockage of airways
- Strangulation: compression and closure of blood vessels and air passages of neck
- Chemical: carbon monoxide and cyanide
- Drowing: breathing in of fluid inside of O2
what is carbon monoxide poisoning (mechanism of cell injury)
carbon monoxide has higher affinity to heme than oxygen so it binds to hemoglobin and prevents transportation of oxygen around body, reducing oxygen carrying capacity of blood. This directly reduces oxygen carrying capacity of blood, which leads to hypoxia
what are free radicals (mechanism of cell injury)
molecules that lost an electron and due to the lost of an electron, they are highly reactive and will bind to anything initiating a chain reaction and causing damage to cell
what are reactive oxygen species (ROS) (mechanism of cell injury)
naturally occurring, formed during mitochondrial respiration. Important in chemical signaling but can lead to pathology in excess
what is air pollution and 6 air pollutants (mechanism of cell injury)
- world’s largest environmental health risk
- 2 types
→ indoor (households heating and cooking)
→ outdoor: increases risk of strokes, heart disease, lung cancer, chronic and acute respiratory diseases (asthma)
- 6 air pollutants: carbon monoxide, particle pollution, ozone, sulfur dioxide, nitrogen dioxide, lead
what is heavy metal toxicity and lead poisoning (mechanism of cell injury)
- heavy metal toxicity: lead, mercury, arsenic, cadmium
- Lead poisoning: exposure in children can result in learning/behavior problems, speech/hearing problems, brain/nervous system injury, slowed growth and development. Most common source is paint in older homes, environment, and work. Toxicity affects central and peripheral nervous system. Treatment can include chelation therapy.
what is ethanol (alcohol) (mechanism of cell injury)
- result in major nutritional deficiencies
- Metabolism occurs primarily in liver.
- Acute alcoholism affects central nervous system
- Chronic alcoholism affects liver (fatty liver, alcoholic hepatitis, cirrhosis) and stomach (gastritis)
- Consumption of alcohol during pregnancy can result in fetal alcohol spectrum disorders
what is necrosis vs apoptosis
- Necrosis: cellular changes after local cell death and lysis; inflammatory changes
- Apoptosis: regulated or programmed cell death; can occur physiologically or pathologically; no inflammatory changes; dysregulated apoptosis can lead to cancer, autoimmune disorders, neurodegenerative disorders, and ischemic injury
what is coagulative and liquefactive necrosis
- Coagulative: occurs in protein-rich organs like heart, kidneys, and adrenal glands
- Liquefactive: associated with injuries to brain tissue in which cells are digested by their own hydrolases
what is caseous, fat, and gangrenous necrosis
- Caseous: described as cheesy and frequently associated with pulmonary tuberculosis
- Fat: associated with process of saponification in which the remaining tissues become chalky and white
- Gangrenous: skin/tissue that have died off due to no oxygen
what are the main intracellular and extracellular cations and anions
- Intracellular: cation is potassium and anions are phosphate and organic ions
- Extracellular: cation is sodium and anions are chloride and bicarbonate
what is sodium
- main extracellular cation
- regulate osmotic forces, nerve impulse conduction, acid-base balance
- normal concentration is 135-145 and is regulated by renin-angiotensin-aldosterone pathway and natriuretic peptides → aldosterone increases Na and water reabsorption in kidney, natriuretic peptide decreases Na and water reabsorption in kidney
what is hypernatremia, symptoms, treatment
- blood sodium >145 → water moves from ICF to ECF and results in intracellular dehydration and shrinkage
- symptoms: affect CNS by causing injury to neurons → lethargy, muscle twitching, confusion, coma, seizures
- treatment: slow correction of electrolyte abnormality
(*Na → brain CNS)
what is hyponatremia, symptoms, treatment
- blood sodium <135 → water moves from ECF to ICF and result in intracellular edema
- symptoms: affect CNS by causing injury to neurons (cerebral edema) → lethargy, confusion, coma, seizures
- treatment: slow correction of electrolyte abnormality
(*Na → brain CNS)
what is potassium
- not same as vitamin K
- normal is 3.5-5.0
- essential for transmission and conduction of nerve impulses, normal cardiac rhythms, muscle contractions
- regulated by Na/K pumps that actively transport K into cells → insulin takes K and moves it in cell, aldosterone, ephinephrine
what is hyperkalemia, symptoms, treatment
- blood potassium >5.0
- seen in kidney impairment due to inability to excrete potassium
- other causes: insulin deficiency, increased dietary intake, excessive cell breakdown (release of intracellular content), certain meds
- symptoms:
→ moderate: tingling of lips, fingers, intestinal cramping/diarrhea, peaked T waves
→ severe: muscle weakness, paralysis, further EKG change, cardiac arrest
- treatment: calcium gluconate, insulin with or without glucose, dialysis
(*potassium → heart)
what is hypokalemia, symptoms, treatment
- blood potassium <3.5
- causes: insufficient dietary intake, increased potassium loss (diarrhea, vomiting, some diuretics), intake of potassium into cells (insulin overdose)
- symptoms: decreased neuromuscular excitability, muscle weakness, cardiac arrhythmia, EKG changes (U waves), cardiac arrest
- treatment: IV or oral replacement of potassium
(*potassium → heart)
what is calcium, where is it stored, functions, what is it related to and regulated by
- majority is stored in bones, only 1% stored within plasma or intracellularly
- Functions to give structure to bones and teeth and assist with blood clotting, hormone secretion, cell receptor function, and muscle contractions
- Concentration of calcium is inversely related to the concentration of phosphate
→ High calcium = low phosphate
→ High phosphate = low calcium
- Regulated by 3 hormones: parathyroid hormone and vitamin D increases Ca levels, calcitonin decreases Ca levels
what is hypercalemia
- Caused by hyperparathyroidism, bone metastasis, excessive vitamin D intake, immobilization, acidosis
- Symptoms: decreased neuromuscular excitability, weakness, kidney stones, constipation, nausea and vomiting, bradycardia and heart block
- Treatment: oral phosphate, IV saline, calcitonin, bisphosphonates, denosumab
(*Calcium → muscles (increase Ca = decreased excitability))
what is hypocalemia
- Caused by inadequate intake or absorption, decreases in parathyroid hormone (thyroidectomy), decreases in vitamin D, blood transfusions, pancreatitis
- Symptoms: increased neuromuscular excitability, muscle spasms and stiffness (tetany), convulsions, chvostek and trousseau signs
- Treatment: calcium replacement, decrease phosphate intake
(*Calcium → muscles (increase Ca = decreased excitability))
what are chvostek and trousseau signs of hypocalcemia
- Chvostek sign: tapping on facial nerve leads to facial twitching
- Trousseau sign: contraction of hand when arterial blood flow is restricted
what is phosphate and hypophosphatemia
- most phosphate is located inside bones – phosphate is used for muscle contraction and energy (ATP)
- Hypophosphatemia:
→ Causes: malabsorption syndromes, poor nutrition, refeeding syndromes
→ Symptoms: symptoms of hypercalcemia, low ATP (hypoxia, bradycardia, coma)
→ Treatment: fix underlying conditions, careful replacement
what is hyperphosphatemia
- Causes: cell breakdown (chemotherapy), inability to excrete (renal failure)
- Symptoms: symptoms of hypocalcemia, calcifications
- Treatment: phosphate binders, dialysis
what is magnesium and hypomagnesemia
- intracellular cation – also stored in muscles and bones and used as a cofactor for intracellular reactions, protein synthesis, nucleic acid stability
- Hypomagnesemia:
→ Caused by malabsorption, poor nutrition; associated with hypocalcemia and hypokalemia
→ Treatment: magnesium replacement
(*magnesium levels being low tend to make calcium and potassium low)
what is hypermagnesemia
- Only seen in renal failure (inability to excrete); associated with hypercalcemia
- Treatment: dialysis, avoid magnesium in diet
what are acids and bases and optimal ranges for pH, PaCO2, and HCO3
- acids donate an H ion and bases accept an H ion
- Optimal pH: 7.35 - 7.45 → pH less than 6.8 = death and pH greater than 7.8 = death
- Optimal PaCO2: 35-45 mmHg
- Optimal HCO3: 22-26
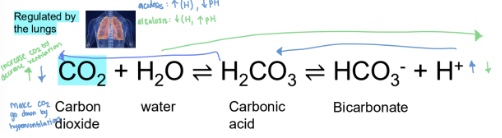
how does the lungs compensate for acidosis and alkalosis
- Compensation for acidosis: increased ventilation → less CO2 → less carbonic acid → less free H → higher pH
- Compensation for alkalosis: decreasing ventilation → more CO2 → more carbonic acid → more free H → lower pH
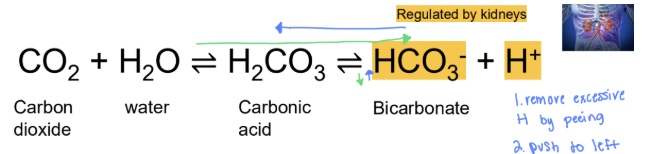
how does the kidneys compensate for acidosis and alkalosis
- Compensation for acidosis: increasing bicarbonate reabsorption and increasing H excretion → more bicarbonate and less H → high pH
- Compensation for alkalosis: increasing H reabsorption and increasing bicarbonate excretion → less bicarbonate and more H → lower pH
what is respiratory acidosis, what is it caused by, manifestations, and treatment
- pH is less than 7.35 and PaCO2 is greater than 45mmHg
- Caused by impaired alveolar ventilation (inability to exhale out CO2) and resultant hypercapnia (high levels of CO2 in blood)
- Treatment: restore adequate ventilation
what is the compensation for respiratory acidosis and manifestations
- Body will compensate by increasing renal bicarbonate reabsorption and eliminating H in urine (renal compensation is not as effective and will be slow)
- Manifestations: headache, restlessness, blurred vision, apprehension, lethargy, muscle twitching, tremors, convulsions, coma
what is respiratory alkalosis, what is it caused by, and treatment
- pH is greater than 7.45 and PaCO2 is less than 35mmHg
- Caused by hyperventilation (breathing out too much CO2) and resultant hypocapnia (take more breaths)
- treatment: treat underlying disorders; sedation with mechanical ventilation may be required
what is the compensation for respiratory alkalosis and manifestations
- Body will attempt to compensate by increasing H reabsorption and eliminating bicarbonate in urine (renal compensation is not as effective and will be slow)
- Manifestations: dizziness, confusion, tingling sensations (paresthesias), convulsions, coma; can have symptoms of hypocalcemia from increased Ca binding to plasma proteins
what is metabolic acidosis, what is it caused by, manifestations, and treatment
- pH is less than 7.35 and bicarbonate is less than 22 mEq/L
- Caused by either a loss of bicarbonate (HCO3) or an increase in non-carbonic acids
- Manifestations: headache, lethargy, confusion, nausea, vomiting, diarrhea, arrythmias, kussmaul respirations
- Treatment: correct underlying disorder, administer buffering solution (sodium bicarb), correct sodium and water deficits
what is the compensation for metabolic acidosis (renal and respiratory)
- renal compensation: excretion of H and reabsorption of bicarbonate
- respiratory compensation: hyperventilation → kussmaul respirations: deep rapid respirations seen in metabolic acidosis
what is metabolic alkalosis, what is it caused by, and manifestations
- pH is greater than 7.45 and bicarbonate is greater than 26
- Caused by either a loss of non-carbonic acids or an increased in bicarbonate
- Manifestations: depends on underlying cause
→ Can have symptoms of hypocalcemia from increasing binding of Ca to plasma proteins
→ Metabolic alkalosis from volume depletion (prolonged vomiting) is associated with weakness, muscle cramps
→ Confusion and convulsions may occur in severe alkalosis
→ Respiratory compensation with slow shallow breathing
what is the compensation for metabolic alkalosis (renal and respiratory) and treatment
- Renal compensation: excretion of bicarbonate and reabsorption of H
- Respiratory compensation: slowing down respirations and retaining CO2
- Treatment: depends on underlying cause; volume replacement with sodium chloride IV solution, potassium replacement
what is transcription, rna splicing, and translation
- Transcription: creation of mRNA from DNA
- RNA splicing: mRNA is matured by removing introns and stitching together extrons
- Translation: mRNA sequence is converted into polypeptide sequence
what is autosomal dominant
- disease is rare and not sex linked
- No generational skipping occurs unless there is a germline mosaicism
- Ex: Huntington's disease
what is autosomal recessive
- not sex linked
- Abnormal allele is recessive, the person must be homozygous to express the disease (dd) → this means that both parents must be carriers (dd or Dd)
- Ex: cystic fibrosis
what is X linked dominant
- Females are more likely to be affected by X-linked dominant disorders than males
- Males have 100% chance of passing to daughter and 0% chance of passing to son
- Females have 50% chance of passing to sons or daughters
- Ex: fragile X syndrome
what is X linked recessive
- Since males only have 1 copy of the X chromosomes, they are significantly more likely to be affected by X-linked recessive disorders
- Affected father will never pass gene to son and always pass to daughter
- Generational skips can occur due to female carriers
- Ex: duchenne muscular dystrophy
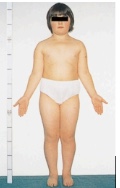
what is turner syndrome, clinical manifestations, and treatment
- Sex-linked aneuploidy; denoted as karyotype 45, X
- Females have only one X chromosome → in turner syndrome every child is female genotype
- Clinical manifestations: Absence of ovaries (sterile), Short stature, Webbing of neck, Widely spaced nipples, High rates of fetal mortality
- treatment: Teenagers receive estrogen replacement therapy to promote secondary sexual characteristics
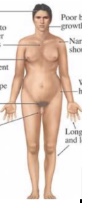
what is klinefelter syndrome
- Sex-linked aneuploidy (will code like male!) (basically male genotype with female characteristics)
- At least 1 Y and 2 or more X chromosomes (X X Y)
- Characteristics: Overall male appearance, Gynecomastia (unintentional development of breast tissue), Small testes, Sparse body hair, May also have extra Y chromosomes
what are the benefits of inflammation
- 1. allowing influx of fluid to the suite (diluting potential toxins), influx and activation of plasma proteins, influx of wbcs to destroy potential pathogens
- 2. plasma protein systems prevent inflammatory response from spreading to healthy tissues
- 3. interacting with adaptive immunity to elicit a more specific response
- 4. allowing for the initiation of wound healing
what are signs of localized inflammation
pain, redness (erythema), heat, swelling (edema), loss of function → local inflammation can also produce exudate or fluid leaking from site of injury
what is the process of phagocytosis
- 1. recognition: Phagocyte must recognize the foreign body through the process of opsonization
- 2. engulfment: The foreign body is surrounded by the phagocyte
- 3. fusion: Phagocyte fuses the foreign body with lysosome to form phagolysosome
- 4. destruction: Lysosome causes destruction of foreign body
what is phagocytosis
- the process by which cells ingest and destroy foreign material
→ mast cells and basophils → allergies and anaphylaxis
→ Eosinophils → parasitic pathogens
→ Monocytes → mature into macrophages
→ Neutrophils → ingest bacteria and cellular debris, turn into purulent exudate
what are the phases of wound healing
1. inflammation
2. reconstruction
3. remodeling and maturation
what is inflammation, the first phase in wound healing
- vasodilation, increased permeability → influx of plasma protein and cells involved in wound healing (platelets, neutrophils, macrophages)
- formation of fibrin mesh (blood clot) which act as a scaffold
- platelets release growth factors to stimulate undamaged cells
- neutrophils and macrophages clean up debris (debridement)
- blood vessels and lymphatics drain away debris
- vascular permeability and dilation reverse back to normal
what is reconstruction, the second phase in wound healing
- Wound begins to heal 3-4 days after initial injury; process continues for 2 weeks
- Fibroblast proliferation and subsequent collagen formation
- Epithelialization: cells from health tissue grow into wound site
- Wound contraction through actions of myofibroblast
- Cellular differentiation in tissues that are capable of regeneration
what is remodeling and maturation, the third phase in wound healing
- Begins several weeks after initial injury and may continue for two years
- Continuation of cellular differentiation (tissue regeneration) and/or scar remodeling
what is active immunity
- immune system activiting
- Antibodies or T-cells that are produced after natural exposure to an antigen or after immunization → typically long lived – longer protection
- Ex: immunization, getting sick (pathogen exposure)
what is passive immunity
- receiving someone else’s immune products
- Pre-formed antibodies or T-cells that are transferred from a donor to a recipient → typically temporary
- Ex: motherhood (breast feeding – IgA in milk) and intravenous immunoglobulin (IgG) injection
what are IgG immunoglobulins
- Most abundant type (80% to 85% of immunoglobulins)
- Account for most of the protective activity against infections
- IgG is transported across placenta for fetal passive immunity (protects babies)
- Only immunoglobulin capable of crossing the placental barrier
what is IgA immunoglobulins
- Has two subclasses:
→ IgA1: predominantly in blood
→ IgA2: predominantly in normal body secretions (saliva, sweat, tears, breast milk)
what is IgE immunoglobulin
- Least concentrated of the immunoglobulin in circulation
- Acts as a mediator of many common allergic responses
- Defends against parasites
- (makes you sick – why you have allergic responses)
what is IgD immunoglobulin
- Information on IgD function is limited
- Concentration is low in the blood
- Located primarily on surface of developing B lymphocytes
- Functions as one type of B-cell antigen receptor
what is IgM immunoglobulin
- Largest of the immunoglobulins (composed of 5 different immunoglobulin molecules)
- First antibody produced during a response to an antigen
- Also synthesized by fetus
- Main antigen produced
- 10 antigen binding sites
what is a type I hypersensitivity
- IgE mediated → typically against environmental antigens (allergens)
- IgE binds to crystalline fragment (Fc) receptors on surface of mast cells; cross linking causes the release of histamine from mast cell degranulation
- Histamine acts on H1 and H2 receptors in the body, which produce multiple different effects
what is the primary exposure and secondary exposure of type I hypersensitivity
- Primary exposure to antigen → B cells find antigen and differentiate into IgE-secreting plasma cells and memory cells, immune system is primed
- Secondary exposure to antigen, IgE antibody reacts to antigen → IgE reacts with mast cells and basophils, degranulation of mast cells and release of histamine
what are cells with H1 and H2 receptors of type I hypersensitivity
- Cells with H1 receptors:
→ Bronchus: (bronchial constriction) - inability to breathe
→ Endothelium: (vasodilation) - lower blood pressure, shock, edema
- Cells with H2 receptors:
→ Gastric parietal cells: (increase secretion of stomach acid) - abdominal pain, nausea
→ Decrease mast cell and basophil degranulation (negative feedback loop)
what is type II hypersensitivity and the 5 mechanisms of injury
- tissue specific reactions → Specific cell or tissue (tissue-specific antigen) are targeted by antibodies
- Five mechanisms of injury by antibodies:
1. antibody trigger complement membrane attack complex
2. antibodies trigger complement, opsonization, and phagocytosis
3. antibodies trigger complement chemotaxis of neutrophils
4. antibody-dependent cell-mediated cytotoxicity
5. antibodies causes target cell malfunction
- Symptoms are based on type of tissue injured and degree of injury
what is type III hypersensitivity
- immune (antigen-antibody) complex mediated → Complexes are formed in circulation and deposited later in vessel walls or extravascular tissues (damage is from complement activation and neutrophil lysosomal enzymes)
- Immune complex clearance:
→ Large complexes can be cleared by macrophages
→ Small complexes can be cleared through liver
→ Intermediate complexes are deposited in tissues – causes problems
what is type IV hypersensitivity
- Mediated by T lymphocytes (aka cell mediated)
- Destruction of tissue is caused directly via toxins from T-cytotoxic cells → Helper T cells also contribute by producing cytokines that recruit phagocytes, especially macrophages
- Ex: acute graph rejections, skin test for TB, contact allergic reactions, some autoimmune diseases (Hashimoto disease, type 1 diabetes)
- Symptoms are based on tissue injured and degree of injury
what are innapropritate immune responses and what is alloiimmune disorder and autoimmune disorder
- Allergies: type I
- Autoimmune disorders: type II, III, IV
- Alloimmune disorders: type II, III, IV
- Immune deficiency
- Alloimmune disorders: immune system forms a response against tissues from another member of same species (attacks beneficial tissue donated to you)
- Autoimmune disorders: breakdown of tolerance – body's immune system begins to recognize self-antigens as foreign
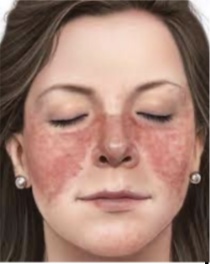
what is systemic lupus erythematosus
- Chronic multisystem inflammatory disease (autoimmune disorder)
- Immune system creates autoantibodies against: nucleic acids, erythrocytes, coagulation proteins, phospholipids, lymphocytes, platelets... (type II)
- Deposition of circulating immune complexes containing anibody against host’s DNA (type III)
-More common in females
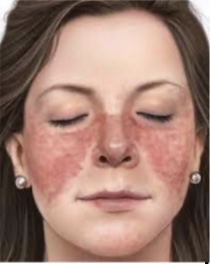
what are the findings that indicates systemic lupus erythematous (need at least 4)
- Facial (malar) rash, discoid (coin) rash, photosensitivity (rash after sunlight), oral or nasopharyngeal ulcers, nonerosive arthritis, serositis, renal disorders, neurologic disorders (seizures, psychosis), hematologic disorders (decreased platelet counts), immunologic disorders, and presence of antinuclear antibodies
- treatment: no treatment but can do nonsteroidal anti-inflammatory, steroids, immunosuppressive agents (all turn off immune system)
what are immune deficiencies and what is primary and secondary
- Immune deficiencies: impaired function of T cells, B cells, phagocytosis, and/or complement
- Primary (congenital): genetic causes like impaired complement
- Secondary (acquired): caused by other illnesses, psychological stress, or physical trauma
- hallmark sign: recurrent severe infections often by opportunistic organisms
what can gonorrhea, viral infections, and microorganisms requiring opsonization
- gonorrhea: complement deficiency
- viral infections: T-cell deficiency
- microorganisms requiring opsonization: B cell and phagocyte deficiencies
what are the stages to an infection
- Incubation period: periods from initial exposure to the onset of first symptoms. Can last from hours or years
- Prodromal stage: occurence of initial symptoms; typically mild with general fatigue and discomfort
- Invasion period: pathogen is multiplying rapidly and spreading systemically; symptoms return and worsen
- Convalescence: symptoms either decline, infection becomes latent, or the person does not survive
what is communicability, infectivity, pathogencitiy, virulence, and toxigenicity of an microorganism
- Communicability: ability to spread from one individual to others and cause disease
- Infectivity: ability to invade and multiply in the host
- Pathogenicity: ability to produce disease
- Virulence: capacity to cause severe disease
- Toxigenicity: ability to produce toxins
what are countermeasures against pathogens
- Environmental infection control methods: Controlling insect population, Providing modern sanitization facilities, Providing clean water
- Antimicrobials
→ Antibiotics: can be bactericidal (kills bacteria) or bacteriostatic (prevents growth of bacteria)
→ Antivirals and antiparasitic agents
- Immunity (active such as vaccines or passive such as human immunoglobulins or IVIG)
what are chemotherapy side effects
- generalized fatigue
- nausea/vomiting (may be severe)
- loss of appetite
- cachexia
- hair loss (alopecia)
- low WBCs count leading to increased risk of infections
- sexual dysfunction
what is the classic cancer 4-stage system
- Stage 1: cancer is confined to organ of origin
- Stage 2: cancer is locally invasive
- Stage 3: cancer is spreading to regional structures like lymph nodes
- Stage 4: cancer has spread to distant sites (metastasis)
what is TMN cancer staging system
- T: tumor
- N: nodes
- M: metastases
what are mechanisms for heat loss
Radiation (heat escapes through radiation)
Conduction (loss of heat through direct contact – laying on something cool)
Convection (loss of heat through gasses or liquids)
Evaporation (sweating)
Vasodilation (blood vessel get bigger)
Decreased muscle tone (muscles dont want to do anything)
Increased pulmonary ventilation (dogs pant to cool down)
Voluntary mechanisms (staying in ac)
what are mechanisms for heat conservation/production (when its too cold)
Vasoconstriction
Skeletal muscle contraction (movement, shivering)
Chemical thermogenesis (fight or flight, epinephrine or norepinephrine)
Chemical reactions of metabolism
Voluntary mechanisms (jackets or indoors)
what are neurological, metabolic, and psychogenic altered levels of consciousness
Neurologic: stokes, seizure, trauma, tumor, intracranial infection, progressive neurologic disease
Metabolic: infection, organ failure, hypoglycemia, drug interactions or overdoses
Psychogenic: underlying psychiatric illness
what is confusion, disorientation, and lethargy (altered levels of consciousness)
Confusion: impaired judgement and decision making; cannot think rapidly or clearly (pt is different from how they normally act)
Disorientation: considered the beginning of loss of consciousness (test person, place, and time since patient will lose orientation to person, place, and time)
Lethargy: mild decrease in arousal. Awakens upon light stimulation but limited spontaneous movements (must put in effort but will cooperate)
what is obtundation and stupor (altered levels of consciousness)
Obtundation: moderate decrease in arousal. Fall asleep unless repeatedly stimulated (very drousey and must keep engaging with)
Stupor: severe decrease in arousal. May open eyes in response to vigorous or noxious stimuli (you r not getting anything from patient)
what is light coma, coma, and deep coma (altered levels of consciousness)
Light coma: unresponsive but with purposeful movements upon stimulation. Eyes may be open or closed (not conscious/awake but will interact if stimulated
Coma: no response to external environment except upon noxious stimulation
Deep coma: unresponsive with no response to any stimuli
what are cheyne-strokes respiration
- abnormal breathing pattern characterized by periodic hyperventilation, hypoventilation, and then apnea
- Caused by bilateral injury to cerebral structures
- Ventilation is ony stimulated once PaCO2 is abnormally increased, slows down, and then stops once PaCO2 drops
- Described as crescendo-decrescendo pattern
- Injury to pons or medulla oblongata may cause hyperventilation, apnea, and agonal breathing patterns
what is brain death and the diagnosis to be declared brain dead
- complete and irreversible cessation of all brain activity including brainstem and cerebellum. Must meet strict diagnosis to be declared brain death
1. absence of confounding variables (no severe underlying metabolic distrubances, no CNS depressant drugs or paralytics, normal core body temperature, normal blood pressure)
2. clinical exam consistent with brain death (no movement, no sign of brainstem activity/absent cranial reflexes, no spontaneous respirations)
3. apnea tests consistent with brain death
4. ancillary tests as desired (EEG, transcranial doppler ultrasound, HMPOA SPECT)
5. if any criteria are not met, then brain death cannot be declared
Once brain death is declared, most states will declare that patient is legally dead
what is brain death confusion
spinal reflex arcs may still be present -> Lazarus sign (reflex arc); patient may continue to have cardiovascular function after declared brain death
what is cerebral death and what do patients typically progress to
- refers to the cessation of cerebral (telencephalon or forebrain) functioning
- In cerebral death, the midbrain and hindbrain are still intact
- Cranial reflexes are present and patient has spontaneous respirations
- Not considered legal death
- Patients typically progress to
→ Persistent vegetative state: spontaneous eye opening without regaining consciousness, cannot follow commands, cannot voluntaryily move, dependent on artificial nutrition
→ Minimally conscious state: occasional brief periods of consciousness and limited movement
what is cerebral death confusion
- locked-in syndrome
- Not brain death
- Inability to communicate either through speech or body movement (is fully aware and know what’s happening but cannot interact with environment)
- CN I-IV are typically preserved (able to blink and move eyes)
- Conscious and cognitive function are intact
- Caused by pontine injuries (central pontine myelinolysis or potine infarcts)
what is delirium and hallmark sign
- Can be considered a type of acute encephalopathy/acute confusional state
- Reversible actue state of dysfunction: typically caused by metabolic disorders (drugs, dehydration, infection, sleep deprivation – no primary deprivation)
- More commonly seen in older adults but greatest risk factor is severity of illness
- Hallmark sign: waxing and waning symptoms (come out of blue then go away)
(*nothing wrong with brain and everything is normal – problem is body and since body isnt working brain is not working)
what is hyperactive delirium and hypoactive delirium and treatment
- Hyperactive delirium: agitation, aggression, restlessness
- Hypoactive delirium: drowsiness, confusion, apathy
- Treatment: supportive care with gentle reorientation, correct of disrupted sleep-wake cycle, review of medications, antipsychotics
what is dementia and alzeheimer
- Progressive non-reversible failure of cerebral functions that causes impairment to arousal and awareness
- Etiologies: degeneration of neurons, compression of brain tissues, atherosclerosis of cerebral vessels, trauma, genetics
- Onset of symptoms is typically gradual and becomes worse with time
- Alzeheimer's: build up of amyloid protein plaques and tau protein bundles
what is hypotonia and hypertonia and its components
- Hypotonia: decreased muscle tone
- Hypertonia: increased muscle tone
→ Spasticity: hyperexcitability of stretch reflexes leading to uncontrolled spastic movements
→ Dystonia: increased voluntary muscle contraction leading to uncontrolled repetitive movements
→ Rigidity: firm and tense muscles that resist movement
what are upper motor neurons and lower motor neurons
Upper motor neurons: brain and spinal cord, injury leads to hypertonia (spasticity, dystonia, ridigity)
Lower motor neurons: spinal nerves and muscles, injury leads to hypotonia (impaired movement, paresis or paralysis)
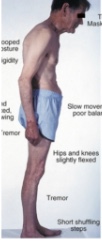
what is parkinson’s disease and treatment
- Exact pathophysiology of parkinson disease is unknown but disease progression leads to loss of neurotransmitter dopamine → Dopamine has a crucial role of inhibiting excess movements in the basal ganglia (part of brain responsible for controlling voluntary movements)
- Treatment: Replace dopamine, Drugs that improve dopamine activity, Rehabilitation, physical therapy, occupational therapy, speech language therapy
(*Progression of Parkinson disease is also associated with dementia)
what are symptoms of parkinson’s disease
→ Loss of dopamine causes hypertonia → hallmark signs: resting tremors, cogwheel rigidity, and bradykinesia (slow movements due to loss of ability to control voluntary movement)
→ Increased tone leads to difficulty walking (hallmark sign: slow shuffling gait)
→ Other common symptoms: frequent drooling, unblinking stare with immobile facial muscles (masked facies)
what is glasgow coma scale and the three factors that are assessed
- assessment of a trauma patient with suspected neurological injury starts with glasglow coma scale (GCS)
- GCS was developed to quantify the severity of a person’s traumatic brain injury and allow for clinical tracking of injury progression
- Three factors that are assessed in GCS: eye opening, verbal response, motor response (all based on patients best response)
what is the score breakdown of glasgow coma scale
- Lowest score on GCS is 3 and highest score is 15
Severe brain injury: 3-8 points
Moderate brain injury: 9-12 points
Minor brain injury: 13-15 points
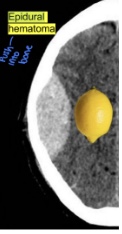
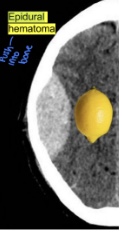
what is a traumatic epidural hematoma
- hemorrhage below the skull but above dura matter → lemon shape
- Typically associated with skull fractures, especially at the site of the temporal fossa (site of meningeal artery and vein)
- Needs emergent evaluation by neurosurgery for potential craniectomy and evacuation of hematoma → surgery is typically indicated
what are the symptoms of traumatic epidural hematoma
- Characteristic symptoms: initial loss of consciousness followed by lucid periods (brief periods where a patient regains consciousness)
→ Following lucid period, symptoms will occur and may progress to nausea, vomiting, lethargy, and coma
- Epidural hematoma expansion is immediately life-threatening