heated under reflux and fractionl distillation
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
why are round or pear shapes flasks used when heating?
they distribute the heat better
why is it better to use a water bath over a bunsen burner?
safer, and bunsens get really hot and the liquids in flask usually have a flow boiling point or are flammable
anti bumping granules use?
control boiling
why do we use bumping granules?
control boiling, and prevents formation of large bubbles which bump out of the flask
what is distillation used for?
seaprate a liquid for a mixture, it is a purification technique
why does water enter the condenser at the bottom and not the top?
to ensure a flow of water, if it entered at the top it'll just drain out, to maximise cooling efficiency
purpose of thermometer in distillation?
reads boiling point of the liquid
when would we use fractional distillation in terms of boiling points?
when separating mixtures with similar boiling points
how are the two mixtures with similar boiling points separated?
both liquids boil and vapours travel up column
higher b.p. substance condenses in column and returns to flask
lower b.p. substances reaches the top of column and into the condenser
set up for oxidation of a primary alcohol to an aldehyde?
distillation

why do we use distillation?
to stop the reaction, the aldehyde leaves the reaction mixture and cant be further oxidised
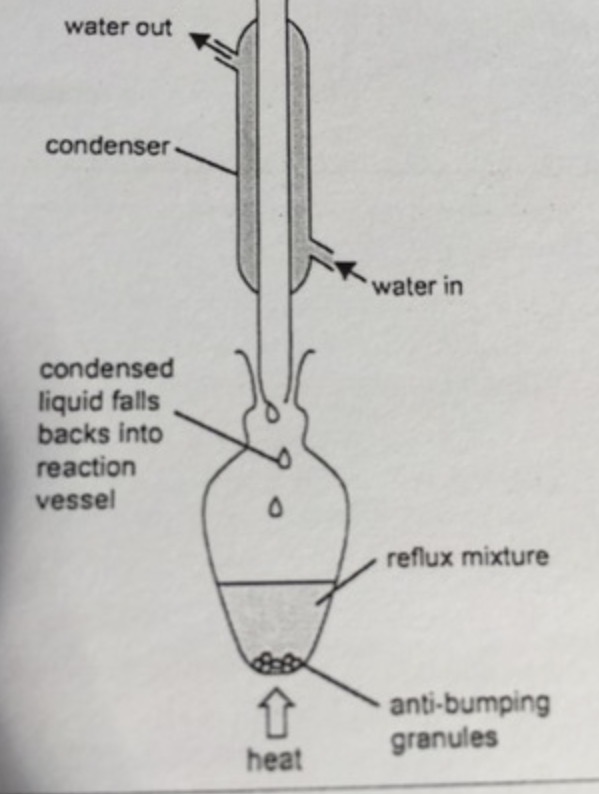
what is heating under reflux?
the process of heating a reaction for a prolonged period of time and condensing the vapors back into the vessel
why is there no thermometer and an open top?
boiling creates gases, therefore high pressure, so lid may pop off and gas goes back into vessel anyway
when do we use reflux?
when we need to heat a mixture containing volatile liquids,
reflux prevents reactants or products from escaping,
helps reaction go to completion (full oxidation of primary alcohols)
draw reflux apparatus