2.1.5 Redox
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What is the rule of assigning an oxidation number to atoms of uncombined elements?
It will always be 0.
e.g. H2, Cl2
What is the rule of assigning an oxidation number to atoms of Group I elements?
They have an oxidation number of +1.
What is the rule of assigning an oxidation number to atoms of Group II elements?
They have an oxidation number of +2.
What is the rule of assigning an oxidation number to fluorine atoms?
They always have an oxidation number of -1.
What is the rule of assigning an oxidation number to oxygen atoms?
They have an oxidation number of -2.
EXCEPT when in a peroxide (H2O2, K2O2, etc), then it is -1
What is the rule of assigning an oxidation number to hydrogen atoms?
They have an oxidation number of +1.
EXCEPT when in a hydride (LiH, MaBH4, etc.), then it is -1.
What is the rule of assigning an oxidation number to simple ions?
It is the charge on the ion.
e.g. Li+, Al 3+
What is the rule of assigning an oxidation number to compound ions (polyatomic ion)?
It is that the sum of all oxidation numbers must equal to the overall charge of the compound.
e.g. SO4 2-
What is the rule of assigning an oxidation number to neutral compounds?
It is that the sum of all oxidation numbers equals to 0.
e.g. H2O
State the oxidation number and name of CoCl2.
ON- Co: +2, Cl: 2(-1)
Name- cobalt chloride
State the oxidation number and name of PO4 3-.
ON- P: +5, O: -8
(+5 - -8 = -3)
Name- phosphorus oxide
Why are Roman numerals used in the name of the compound to indicate their oxidation numbers?
This is because these elements do not have an oxidation number rule.
e.g. transition metals, B, C, N, etc.
State the oxidation number and name of FeO.
ON - Fe: +2, O: -2
Name - Iron (II) oxide
*Roman numeral indicates its oxidation number.
State the oxidation number and name of Fe2O3.
ON- Fe: +3, O: -2
Name- Iron (III) oxide
State the oxidation number and name of HNO3.
ON- H: +1, N: +5, O: -6
(It is a neutral compound so overall charge is 0, N has a variable ON)
Name- Nitric (V) acid
What are definitions of oxidation and reduction in terms of electron transfer?
Oxidation - LOSS of electrons
Reduction - GAIN of electrons
(OIL RIG)
What are the definitions of oxidation and reduction in terms of changes in oxidation number?
An element is oxidised when its oxidation number increases.
An element is reduced when its oxidation number decreases.
Define oxidising agent.
A reagent that oxidises (takes e- from) another species. The oxidising agent itself is reduced in the process.
Define reducing agent.
A reagent that reduces (gives e- to) another species. The reducing agent itself is oxidised in the process.
What is the oxidising and reducing agent in Mg + 2HCl → MgCl2 + H2 .
Oxidised: Mg, lost electrons
Reduced: H+ , gained electrons
Oxidising agent: H+
Reducing agent: Mg
What are the writing frames to follow to describe redox reactions in terms of electron transfer or oxidation number?
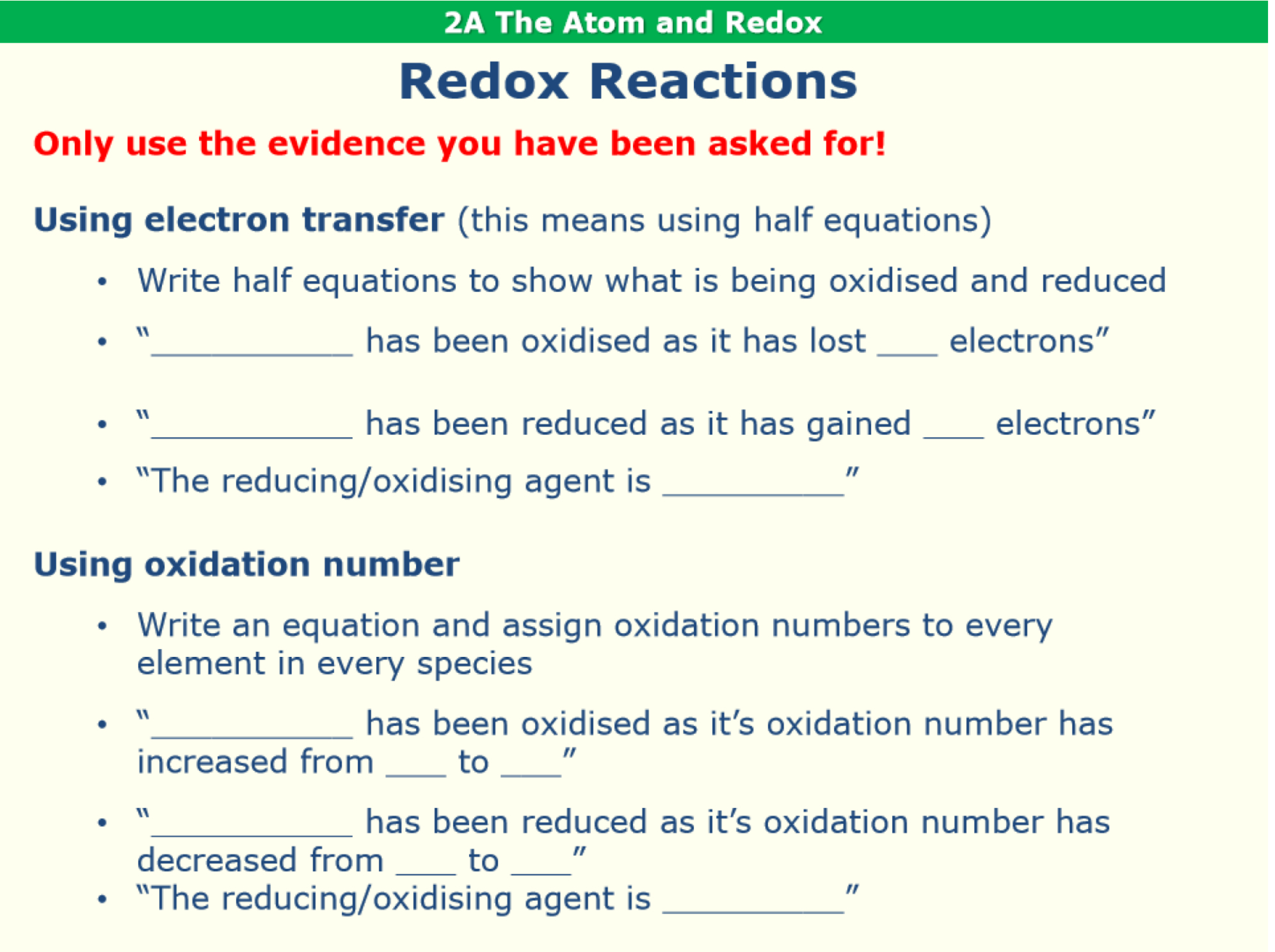
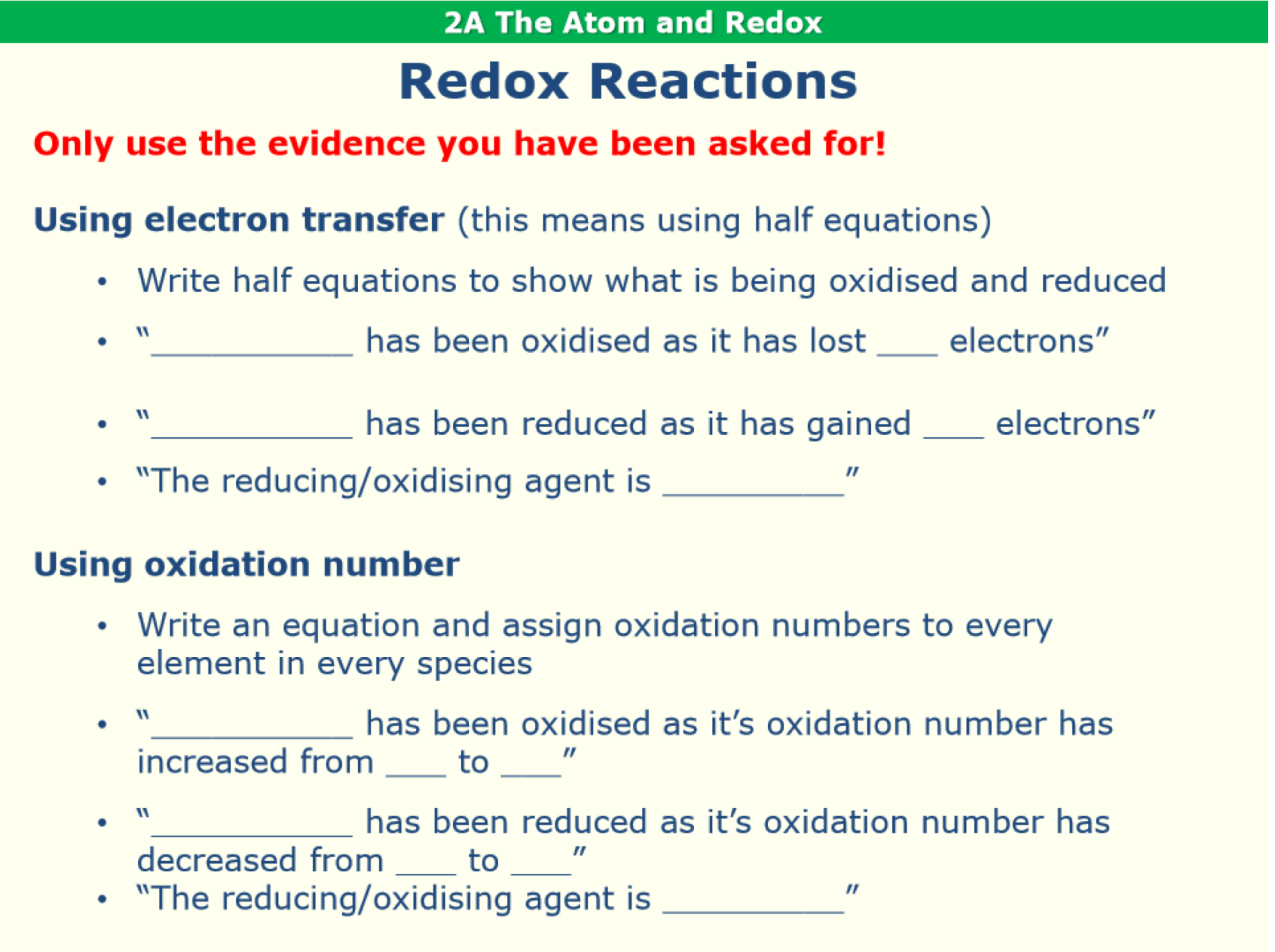
Using oxidation numbers, describe the redox behaviour of the following reaction. Identify the reducing agent.
Cl2 (aq) + 2KI (aq) → 2KCl (aq) + I2 (aq)
Cl2 (aq) + 2KI (aq) → 2KCl (aq) + I2 (aq)
0 +1 +1 +1 +1 0
I has been oxidised as its oxidation number increased from 01 in KI to 0 in I2.
Cl2 has been oxidised as its oxidation number has decreased from 0 in Cl2 to -1 in KCl.
The reducing agent is iodide ions.
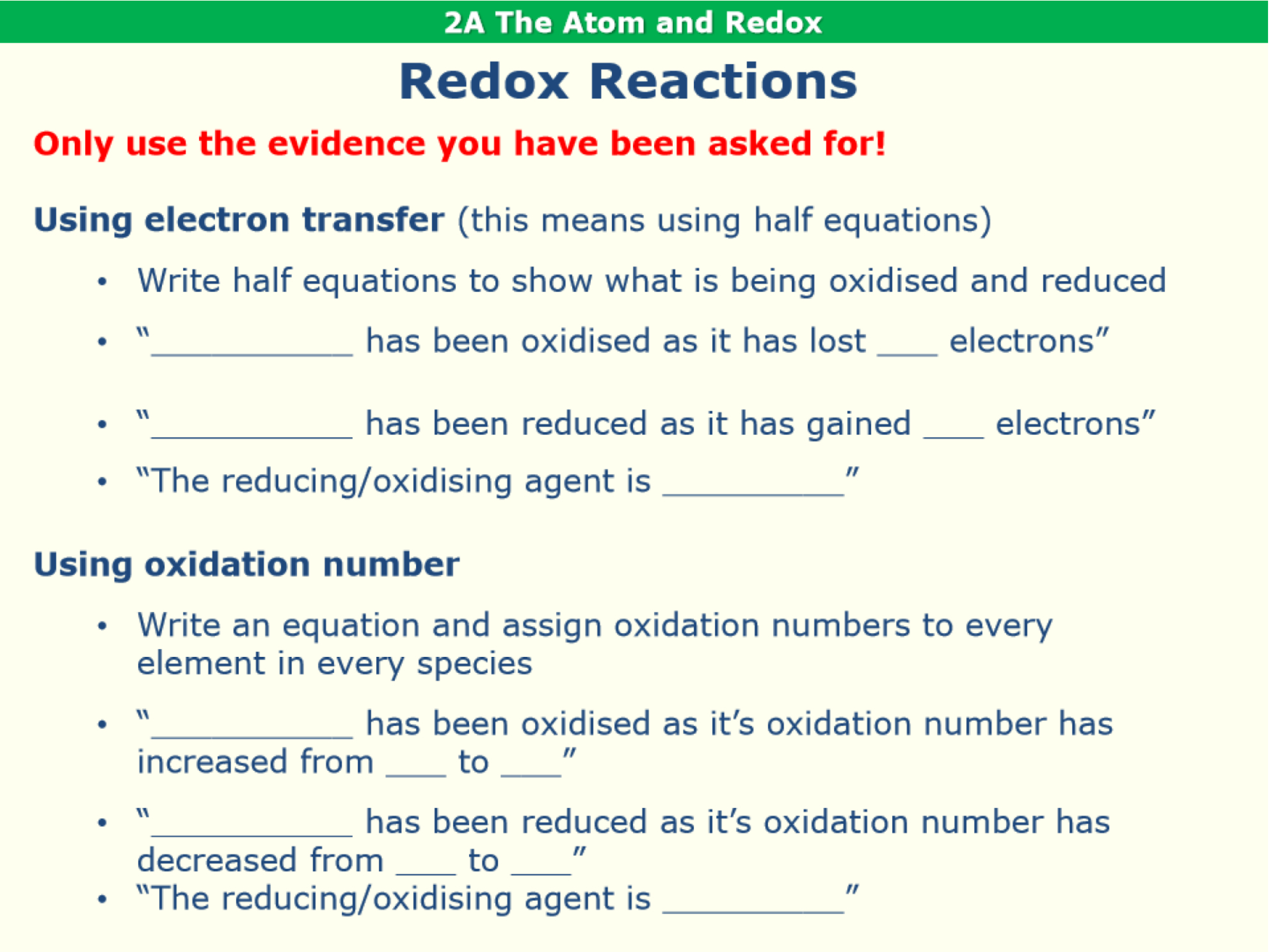
Using electron transfer, explain the redox behaviour of the reaction of magnesium metal with hydrochloric acid. Identity the oxidising agent.
(this means using half equations)
Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
0 +1 -1 +2 -1 0
Ox: Mg → Mg 2+ + 2e-
Red: 2H+ + 2e- → H2
Magnesium has been oxidised as it has lost 2 electrons.
Hydrogen has been reduced as it has gained 1 electron.
The oxidising agent is H+.