CH 221z: Electron and Molecular Geometry (VSEPR)
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
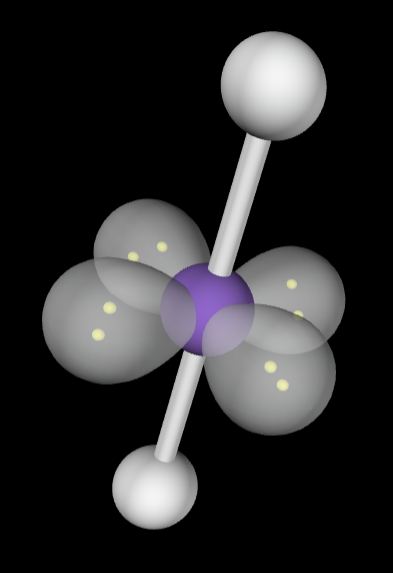
2 bonding groups
Electron Geometry: Linear
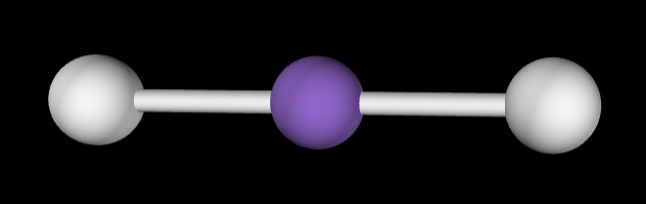
3 bonding groups
Electron Geometry: Trigonal planar
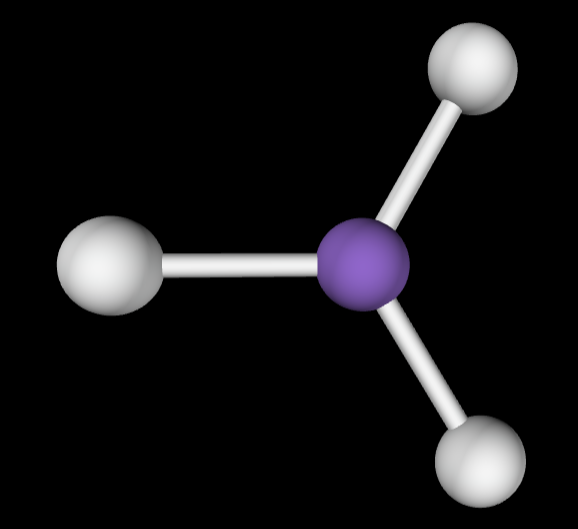
4 bonding groups
Electron Geometry: Tetrahedral
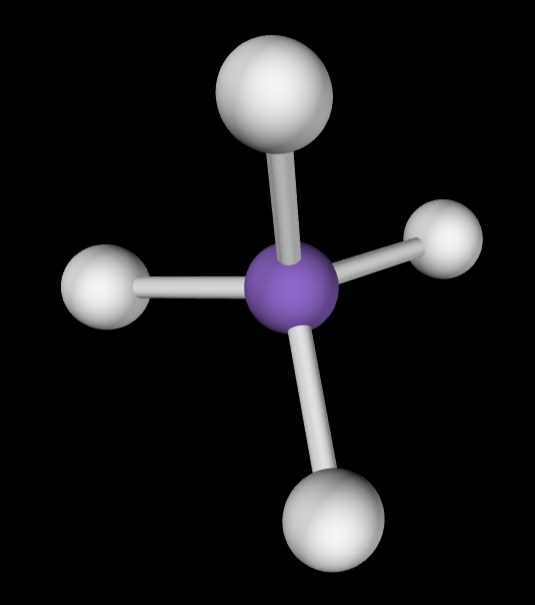
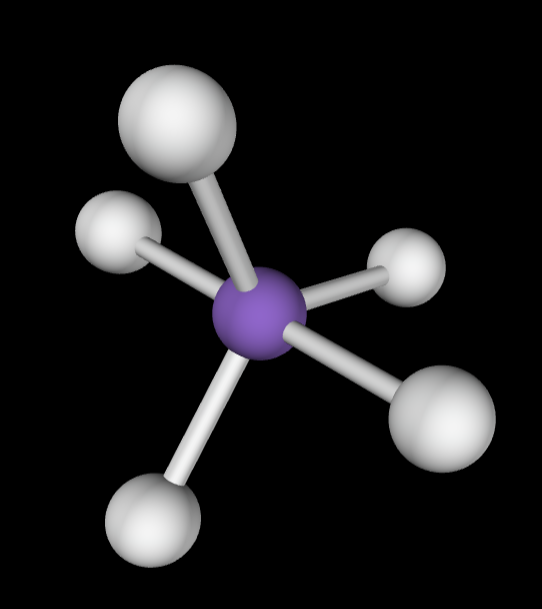
5 bonding groups
Electron Geometry: Trigonal bipyramidal
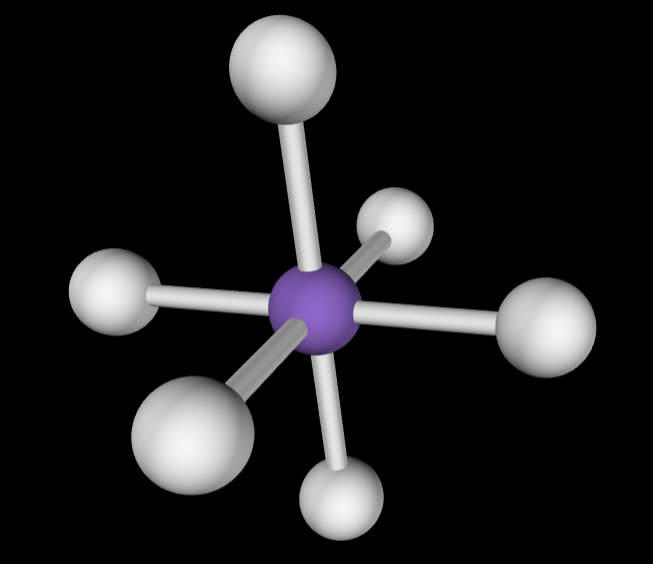
6 bonding groups
Electron Geometry: Octahedral
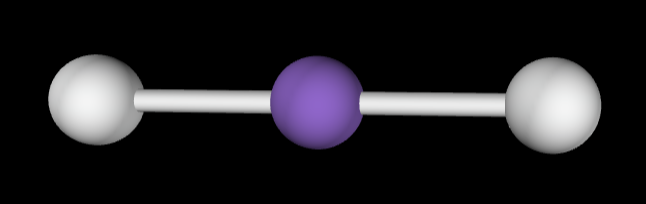
→2 bonding groups
→no lone pairs
Molecular Geometry: Linear
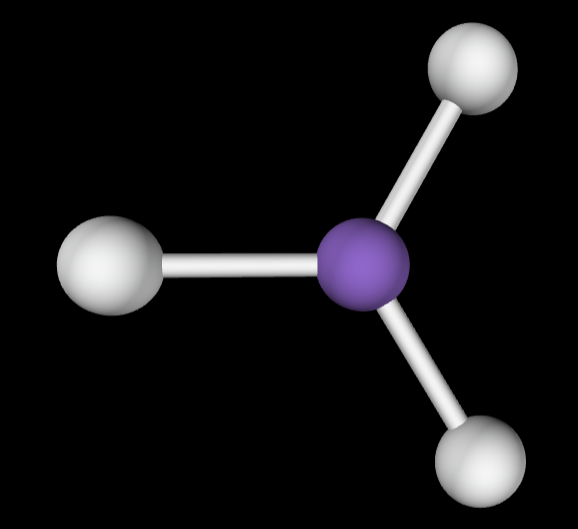
→3 bonding groups
→No lone pairs
Molecular Geometry: Trigonal planar
→3 bonding groups
→1 lone pair
Molecular geometry: Bent
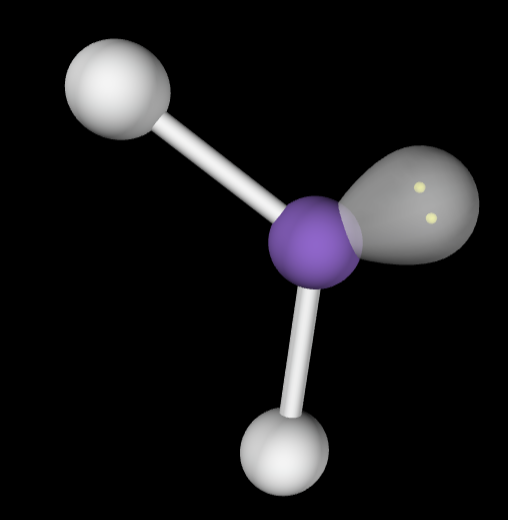
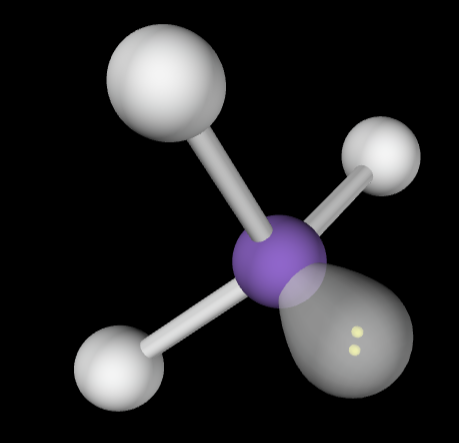
→4 bonding groups
→No lone pairs
Molecular Geometry: Tetrahedral
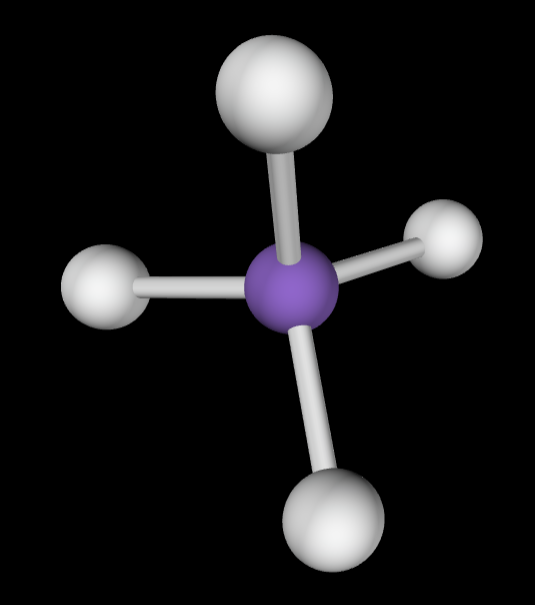
→4 bonding groups
→1 lone pair
Molecular Geometry: Trigonal pyramidal
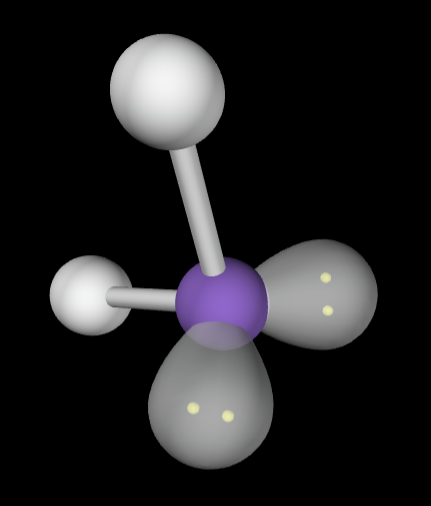
→4 bonding groups
→2 lone pairs
Molecular geometry: Bent
→5 bonding groups
→No lone pairs
Molecular Geometry: Trigonal bipyramidal
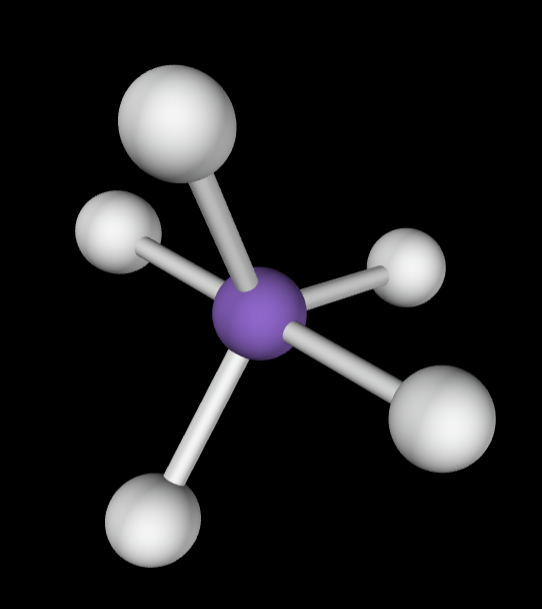
→5 bonding groups
→1 lone pair
Molecular Geometry: See-saw
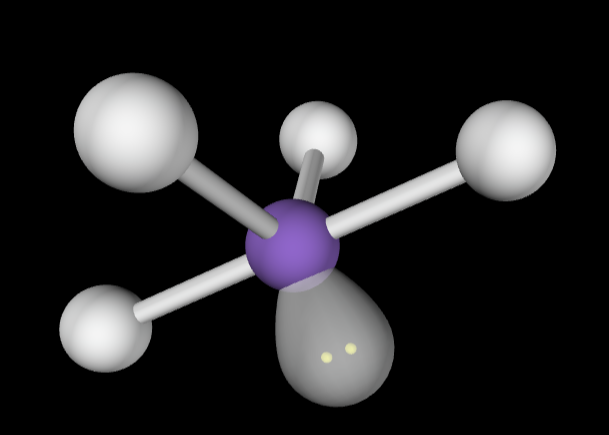
→5 bonding groups
→2 lone pairs
Molecular Geometry: T-Shaped
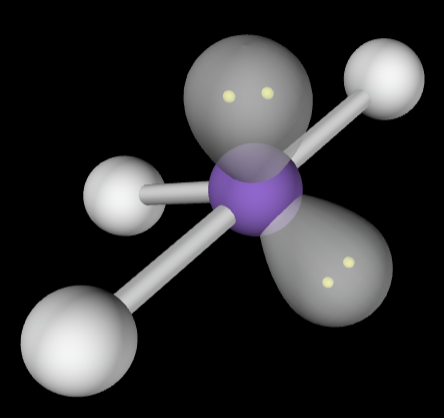
→5 bonding groups
→3 lone pairs
Molecular Geometry: Linear
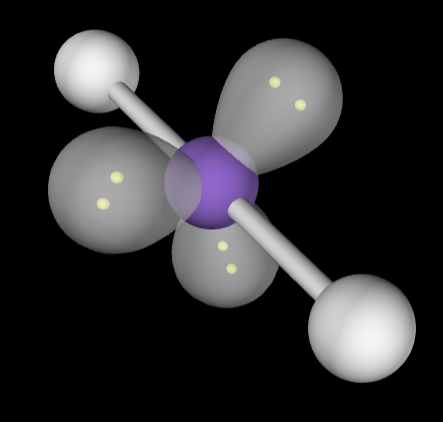
→6 bonding groups
→No lone pairs
Molecular Geometry: Octahedral
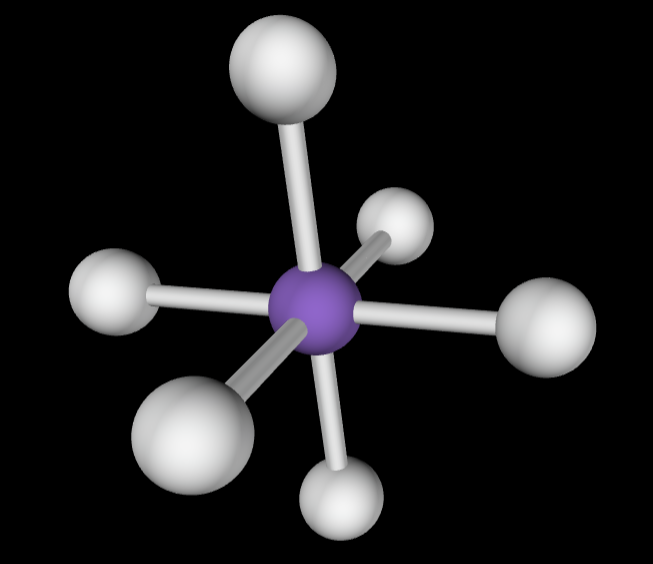
→6 bonding groups
→1 lone pair
Molecular Geometry: Square pyramidal
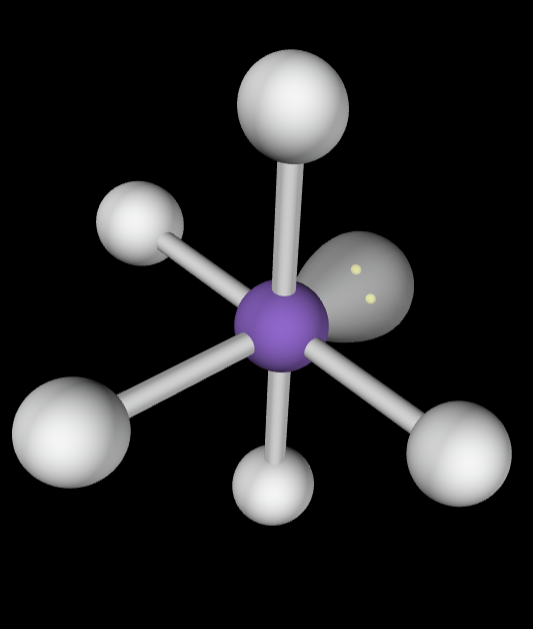
→6 bonding groups
→2 lone pairs
Molecular Geometry: Square planar
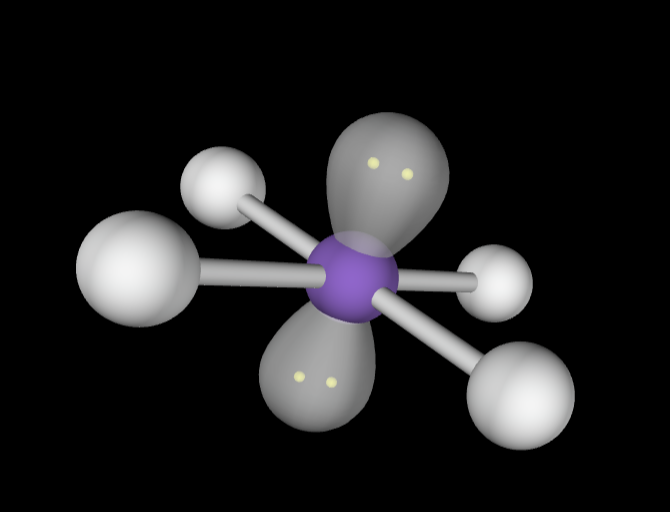
→6 bonding groups
→3 lone pairs
Molecular Geometry: T-Shaped
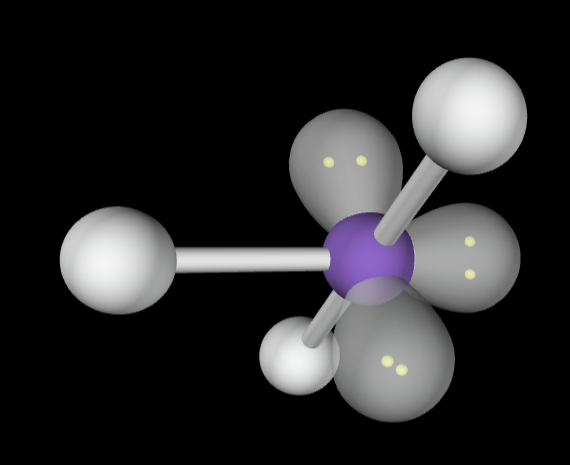
→6 bonding groups
→4 lone pair
Molecular Geometry: Linear